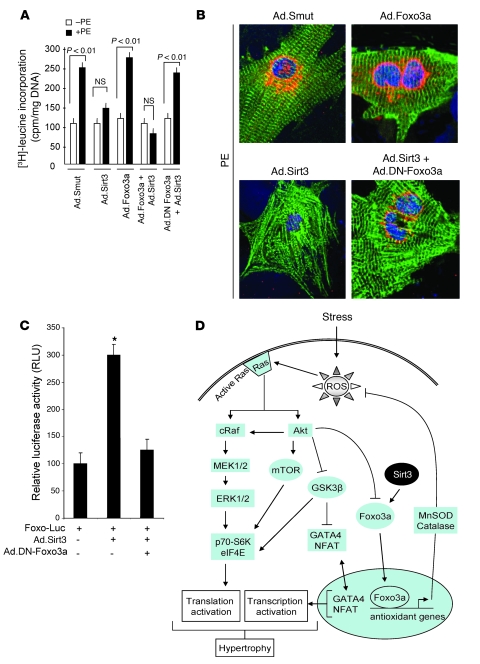
Figure 10. A DN-Foxo3a eliminates the antihypertrophic effect of Sirt3.
Cardiomyocytes were prepared from neonatal Sirt3-KO mice. They were infected with different combinations of adenoviruses as indicated and then stimulated with PE (20 μM) for 48 hours. (A) Measurement of [3H]-leucine incorporation into total cellular protein (mean ± SEM, n = 5; P < 0.01). (B) Immunostaining of cells with anti-ANF (red) and anti–α-actinin (green) antibodies. Positions of nuclei were determined by DAPI staining (blue). Original magnification, ×1,000. (C) The DN-Foxo3a inhibits the activity of endogenous Foxo3a. Cardiomyocytes were overexpressed with a Foxo3a responsive/luciferase reporter plasmid and viruses synthesizing Sirt3 or DN-Foxo3a in different combinations, as indicated. The luciferase activity was determined 48 hours after transfection. All values are normalized to protein content of the cell (mean ± SEM, n = 4; *P < 0.01). The DN-Foxo3a was capable of blocking Sirt3-dependent activation of Foxo-promoter activity. (D) Scheme illustrating signaling pathways modified by Sirt3 to block the cardiac hypertrophic response. Sirt3 levels are elevated during stress of cardiomyocytes, which deacetylates Foxo3a and traps it inside the nucleus to enhance the transcription of Foxo-dependent antioxidant genes, MnSOD and Cat. Increased expression of MnSOD and catalase suppresses ROS levels generated by stress stimuli. Because ROS is the second messenger of hypertrophic signaling pathways, suppression of ROS levels shuts down major signaling pathways involved in activation of transcription and translation events contributing to the cardiac hypertrophic response.