Abstract
A large number of plastid proteins encoded by the nuclear genome are posttranslationally imported into plastids by at least two distinct mechanisms: the Toc159-dependent and Toc132/Toc120-dependent pathways. Light-induced photosynthetic proteins are imported through the Toc159-dependent pathway, whereas constitutive housekeeping plastid proteins are imported into plastids through the Toc132/Toc120 pathway. However, it remains unknown which features of the plastid protein transit peptide (TP) determine the import pathway. We have discovered sequence elements of the Rubisco small subunit TP (RbcS-tp) that play a role in determining import through the Toc159-dependent pathway in vivo. We generated multiple hybrid mutants using the RbcS-tp and the E1α-subunit of pyruvate dehydrogenase TP (E1α-tp) as representative peptides mediating import through the Toc159-dependent and Toc159-independent pathways, respectively. Import experiments using these hybrid mutants in wild-type and ppi2 mutant protoplasts revealed that multiple sequence motifs in the RbcS-tp independently contribute to Toc159-dependent protein import into chloroplasts. One of these motifs is the group of serine residues located in the N-terminal 12-amino acid segment and the other is the C-terminal T5 region of the RbcS-tp ranging from amino acid positions 41 to 49. Based on these findings, we propose that multiple sequence elements in the RbcS-tp contribute independently to Toc159-dependent import of proteins into chloroplasts.
The plastid is a crucial organelle in plant cells. It plays a role in critical cellular processes such as photosynthesis, ATP generation, amino acid metabolism, and synthesis of fatty acids and lipid components. Accordingly, a large number of proteins are required for all these activities in plastids. Some of these proteins are encoded by the chloroplast genetic system and are translated in the plastids. However, most plastid proteins (over 90%) are encoded by the nuclear genome and are imported into plastids from the cytosol posttranslationally (Kessler and Schnell, 2006; Jarvis, 2008).
Most plastid interior proteins that undergo posttranslational import from the cytosol contain a cleavable N-terminal targeting signal, a transit peptide (TP), of 50 to 70 amino acid residues (Jarvis, 2008; Lee et al., 2008). However, recently, some plastid interior proteins have been identified that do not have the N-terminal canonical TP (Miras et al., 2002, 2007; Nada and Soll, 2004). The long TP consists of multiple domains or motifs that encode information for preprotein import into plastids (von Heijne et al., 1989; Pilon et al., 1995; Rensink et al., 2000; Lee et al., 2006, 2008). The preproteins transit through the cytosol as unfolded protein. During passage through the cytosol, they may form a complex with heat shock proteins, such as Hsp70 and Hsp90, and guidance factors such as 14-3-3 (May and Soll, 2000; Qbadou et al., 2006). However, 14-3-3 may not be essential for the targeting of these proteins to chloroplasts (Lee et al., 2002, 2006; Nakrieko et al., 2004). To cross the two envelope membranes, the TP interacts with components of the Toc and Tic complexes located at the outer and inner envelopes of chloroplasts, respectively (Jarvis, 2008). These include members of the Toc159 family, Toc33/Toc34, Toc75, and Tic20. At the late stage or after translocation, the TP is recognized and cleaved off by stromal processing peptidases (Richter and Lamppa, 1999; Chen and Li, 2007).
Despite extensive study of the TPs, it is not fully understood how the information encoded in these peptides is decoded by the plastid protein import machinery. TPs display some degree of similarity in their amino acid composition, including a higher content of Ala, Gly, and the hydroxylated amino acids Ser and Thr, and a lack of acidic amino acids (von Heijne et al., 1989; Bruce, 2001; Zhang and Glaser, 2002). However, it is clear that the entire family of TPs, termed the transit peptidome, cannot be represented by a single consensus sequence. Growing evidence has pointed to a functional classification of TPs. The first indication is that the transit peptidome may be classified into two groups: Toc159-dependent and Toc159-independent TPs (Ivanova et al., 2004; Kubis et al., 2004; Smith et al., 2004). The TPs that confer Toc159 dependence in protein import are typically used by light-induced photosynthetic proteins, whereas Toc159-independent TPs are used by nonphotosynthetic and housekeeping proteins (Kessler and Schnell, 2006). This was clearly demonstrated in the ppi2 mutant that has a T-DNA insertion in atTOC159 (Smith et al., 2004). In accord with this observation, the expression of atTOC159 is high in young and photosynthetic tissues whereas atTOC132 and atTOC120 are expressed uniformly in all plant tissues at low levels (Kubis et al., 2004). In addition, in nonphotosynthetic tissues, such as roots, the mRNA level of atTOC132 or atTOC120 is much higher than that of atTOC159. These results are consistent with the hypothesis that TPs may contain sequence motifs that determine the targeting pathway. However, the sequence information that confers Toc159 dependence or Toc132/120 dependence on these proteins during protein import remains unknown. In addition, Lee et al. (2008) recently demonstrated that the transit peptidome may be divided into several groups based on critical sequence motifs present in the TP. However, the role of the sequence motifs embedded in the TPs is not entirely clear yet with respect to translocation through the envelope membranes and also to the molecular machinery that recognizes these sequence motifs. Furthermore, the sequence information that confers Toc159 dependence or Toc132/120 dependence in protein import on these proteins remains unknown.
The Rubisco small subunit (RbcS) and E1α TPs (RbcS-tp and E1α-tp) confer Toc159 dependence and Toc159 independence in protein import into chloroplasts, respectively (Smith et al., 2004). In this study, using these two TPs, we have determined the RbcS-tp sequence motifs that confer Toc159 dependence. Here, we have demonstrated that Toc159-dependent protein import is mediated independently by multiple sequence motifs: one of them is the group of Ser residues located in the N-terminal 12-amino acid segment and the other is in the C-terminal region ranging from amino acid positions 41 to 49.
RESULTS
RbcS-tp:GFP and E1α-tp:GFP Are Imported into Chloroplasts through Toc159-Dependent and -Independent Pathways, Respectively
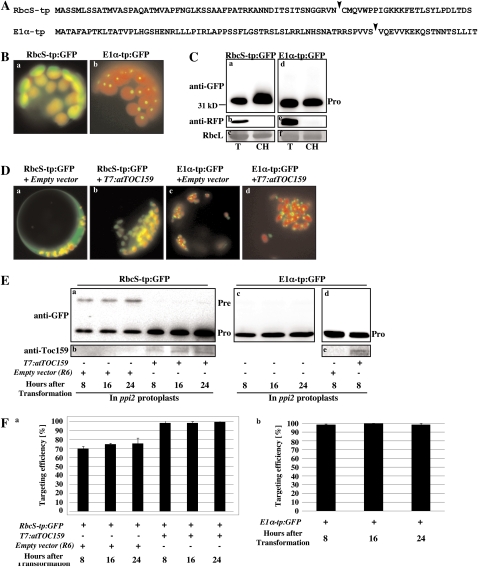
To examine the molecular mechanisms of the Toc159-dependent and -independent pathways, we utilized two reporter proteins, RbcS-tp:GFP (formerly RbcS-nt:GFP) and E1α-tp fused to GFP (E1α-tp:GFP; Fig. 1A). RbcS-tp:GFP is a chimeric protein composed of the 79 N-terminal amino acids of the RbcS, which has been characterized in detail as a model cargo for the Toc159-dependent pathway (Lee et al., 2003; Smith et al., 2004), and GFP. E1α is the E1α-subunit of pyruvate dehydrogenase, which is a constitutive nonphotosynthetic plastid protein accumulated normally in ppi2 mutants (atTOC159 knockout) as in wild-type plants (Smith et al., 2004). Moreover, the E1α TP (1α-tp) interacts preferentially with atToc132 over atToc159 (Ivanova et al., 2004; Smith et al., 2004), indicating that E1α may be imported into chloroplasts by a Toc159-independent, but Toc132/120-dependent, pathway. To confirm this hypothesis, the N-terminal 80 amino acid residues (the TP of 61 amino acid residues + an extra region of 19 amino acid residues from mature portion) of E1α was fused to GFP to create E1α-tp:GFP (Fig. 1A) and the resulting construct was introduced into leaf tissue protoplasts by polyethylene glycol-mediated transformation (Jin et al., 2001; Kim et al., 2001). GFP signal was detected in the chloroplasts by fluorescent microscopy (Fig. 1B). Moreover, this GFP fusion protein was processed to the mature forms, which are copurified with isolated chloroplast fractions, indicating that E1α-tp:GFP is imported into chloroplasts (Fig. 1C). However, in contrast to the diffuse GFP pattern seen with RbcS-tp:GFP, the processed form of E1α-tp:GFP produced a punctate staining pattern with weak diffuse signal within the chloroplast, indicating that the processed form of E1α-tp:GFP may have a tendency to form aggregates in the stroma (Fig. 1B). The observation that E1α-tp:GFP produced an aggregate pattern in both wild-type and ppi2 protoplasts (Fig. 1B) differs from a previous observation made by Smith et al. (2004). This discrepancy may be due to differences in the lengths used as targeting signals between the two studies. In the previous study by Smith et al. (2004), the N-terminal 65-amino acid fragment consisting of the 61-amino acid TP and a four-amino acid fragment from mature portion of E1α was used. In contrast, in this study, the N-terminal 80-amino acid fragment of the same protein was used to generate the fusion protein E1α-tp:GFP and thus contained an extra 15 amino acid residues as compared to that of Smith et al. (2004). It is possible that the extra N-terminal region of E1α employed in this study caused aggregation of GFP reporter proteins in the stroma of chloroplasts. Consistent with this explanation, E1α49/RbcS-tp:GFP and E1α59/RbcS-tp:GFP, in which the extra mature region of E1α was replaced with the corresponding region of RbcS-tp, did not produce aggregate patterns (see below in Fig. 4).
Figure 1.
Protein import of RbcS-tp:GFP and E1α-tp:GFP in wild-type and ppi2 mutant protoplasts. A, Sequences of RbcS-tp and E1α-tp. TP cleavage sites were predicted by TargetP (Emanuelsson et al., 2000) and indicated by arrowheads. B, Images of RbcS-tp:GFP and E1α-tp:GFP. Protoplasts from wild-type plants were transformed with RbcS-tp:GFP and E1α-tp:GFP and images were taken at 8 h after transformation. C, Western-blot analysis of RbcS-tp:GFP and E1α-tp:GFP. Protoplasts were transformed with RbcS-tp:GFP or E1α-tp:GFP together with RFP, and intact chloroplasts were purified using a percoll gradient. Protein extracts were prepared from intact purified chloroplasts and analyzed by western blotting using anti-GFP or anti-RFP antibodies. Total protoplast extracts were also included in the analysis. RFP was used as a representative of cytosolic proteins and the Rubisco large subunit stained with Coomassie Brilliant Blue was used as a control for chloroplast purification. Pro, Processed forms; T, total protein extracts; CH, extracts from intact purified chloroplasts. D, Images of RbcS-tp:GFP and E1α-tp:GFP in ppi2 mutant protoplasts. Protoplasts from ppi2 plants were transformed with RbcS-tp:GFP or E1α-tp:GFP together with R6 (an empty expression vector) or T7:atTOC159. Images were taken 8 h after transformation. E, Protein import into ppi2 protoplasts. RbcS-tp:GFP and E1α-tp:GFP were transformed into ppi2 protoplasts together with R6 (empty expression vector) or T7:atTOC159, and protein extracts from transformed protoplasts were analyzed by western blotting using anti-GFP and anti-Toc159 antibodies at the indicated time points after transformation. Pre, Precursor forms. F, Quantification of protein imported into ppi2 chloroplasts. The import experiments were performed three times (Fig. 1E) and the data show the average import efficiencies at each time point (n = 3; error bar, sd). The import efficiency is expressed as the percentage of the amount of the processed form divided by the total amount of expressed protein.
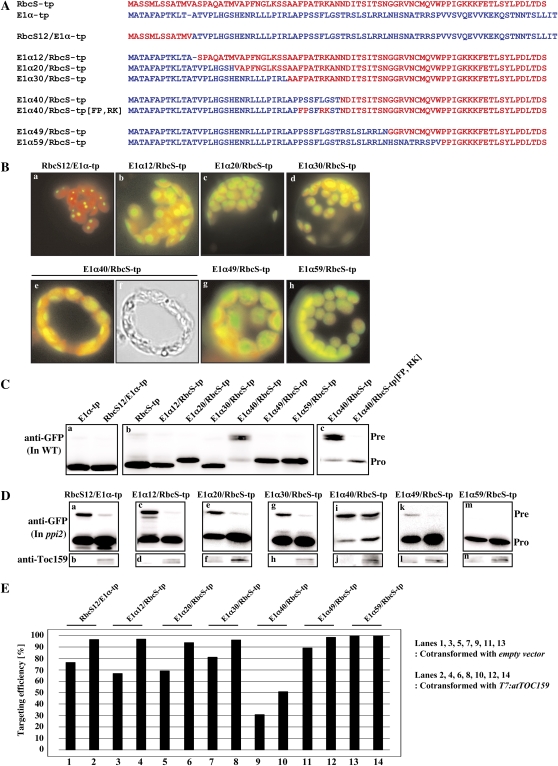
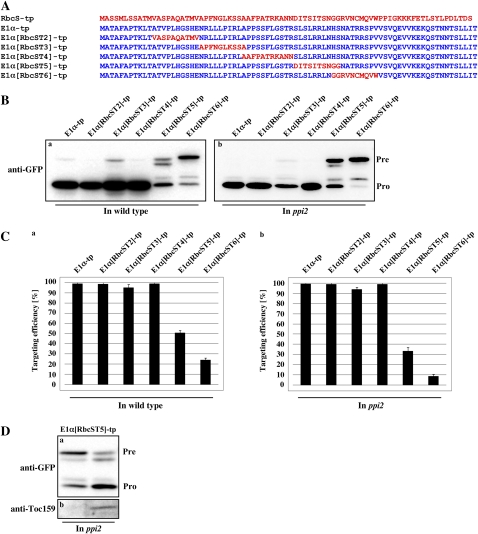
Figure 4.
Toc159 dependence of RbcS-tp is mediated independently by multiple regions. A, Amino acid sequences of hybrid TPs of E1α-tp and RbcS-tp. The N-terminal 12-amino acid residues of RbcS-tp and E1α-tp were swapped to produce RbcS12/E1α-tp and E1α12/RbcS-tp. In addition, the 20-, 30-, 40-, 49-, or 59-amino acid N-terminal fragment of RbcS-tp was replaced by the corresponding N-terminal E1α-tp fragment. B, Import experiments with hybrid TPs. Protoplasts were transformed with the indicated constructs and the import of these reporter proteins was examined directly by observing GFP signals with a fluorescent microscope. In the case of E1α40/RbcS-tp, transformed protoplasts were immunostained with anti-GFP antibody followed by fluorescein isothiocyanate-labeled secondary antibody because it gave no GFP signal. C and D, Western-blot analysis of import experiments. Protoplasts from wild-type (WT; C) or ppi2 mutant (D) plants were transformed with the plasmids encoding swapping mutants alone or together with T7:atTOC159, and total protein extracts were analyzed by western blotting using anti-GFP and anti-Toc159 antibodies. Pre, Precursor forms; Pro, processed forms. E, Quantification of targeting efficiency. The import efficiency is expressed as the percentage of the amount of the processed form divided by the total amount of expressed protein.
To test whether or not proteins are imported into chloroplasts by the Toc159-dependent pathway, we took advantage of the existence of ppi2 mutants (Smith et al., 2004). Protoplasts from ppi2 mutants were transformed with RbcS-tp:GFP or E1α-tp:GFP and with T7:atTOC159 or a control empty vector. At 8 h after transformation, E1α-tp:GFP was efficiently imported into chloroplasts in ppi2 protoplasts (Fig. 1, D [section c] and E [section d]). Moreover, coexpression of T7:atToc159 did not affect the import efficiency, indicating that E1α-tp:GFP is imported into chloroplasts in an atToc159-independent manner (Fig. 1, D and E). As a control, we examined the import efficiency of RbcS-tp:GFP in ppi2 protoplasts. In ppi2 protoplasts, about 30% of RbcS-tp:GFP remained in the precursor state (Fig. 1, E and F). In wild-type protoplasts, there was a nearly undetectable level of RbcS-tp:GFP precursor (Fig. 1C). Furthermore, in ppi2 protoplasts, coexpression of T7:atToc159 reduced the precursor levels significantly to less than 5% of total RbcS-tp:GFP protein (Fig. 1, E and F). These results clearly indicate that RbcS-tp:GFP and E1α-tp:GFP are imported into chloroplasts by the Toc159-dependent and Toc159-independent pathways, respectively.
Identification of Sequence Motifs in the E1α-tp Required for Efficient Chloroplast Targeting
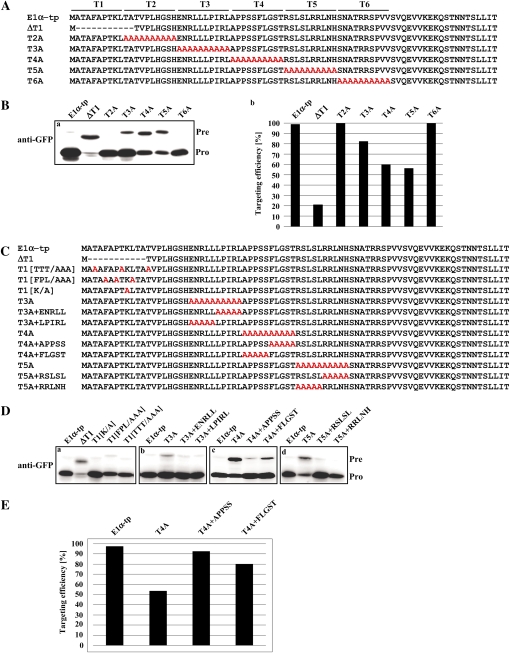
We have previously identified RbcS-tp sequence motifs required for protein import into chloroplasts (Lee et al., 2006). Here, in a similar approach, we identified sequence motifs in the E1α-tp critical for protein import into chloroplasts. Serial Ala scanning mutants, E1α[T2A], E1α[T3A], E1α[T4A], E1α[T5A], and E1α[T6A], were generated by replacing the second, third, fourth, fifth, and sixth 10-amino acid blocks, respectively, with Alas (Fig. 2A). Ala substitution of the first 10-amino acid block produced an undetectable level of protein. Thus, we next generated E1α[ΔT1] that contained a deletion of the 11 N-terminal amino acids (Fig. 2A). All of the mutant TPs were fused to the N terminus of GFP, and the resulting plasmids were transformed into protoplasts. At 8 h after transformation, import efficiency was monitored by immunoblotting with anti-GFP antibody. E1α[T2A]-tp:GFP and E1α[T6A]-tp:GFP were imported into chloroplasts as efficiently as wild-type E1α-tp (Fig. 2B, sections a and b). E1α[T3A]-tp showed a mild defect in chloroplast targeting, whereas E1α[ΔT1]-tp, E1α[T4A]-tp, and E1α[T5A]-tp were severely affected in delivering proteins to chloroplasts, indicating that the T1, T3, T4, and T5 segments contain critical sequence motifs for import of proteins into the chloroplast (Fig. 2B, sections a and b). The N-terminal 12-amino acid segment of E1α-tp consists of four Alas, three Thrs, three hydrophobic residues (Phe, Pro, and Leu), and one Lys residue. To investigate which of the residues in the T1 segment is important for chloroplast targeting, we constructed mutants, E1αT1[TTT/AAA], E1αT1[FPL/AAA], and E1αT1[K/A], that contained Ala substitution of the three Thrs (TTT), the three hydrophobic amino acids (FPL), and the single Lys (K), respectively (Fig. 2C). These mutants were fused to GFP and then we examined their ability to deliver protein to chloroplasts. Unexpectedly, all of these mutants were efficiently targeted to chloroplasts (Fig. 2D, section a), raising the possibility that the length, but not the specific amino acid sequence, of the TP is critical for targeting. In previous studies, the N-terminal hydrophobic amino acids have been shown to be conserved in the TPs and play a critical role in protein import into chloroplasts (Lee et al., 2006, 2008). However, this result raised the possibility that the hydrophobic residues in the T1 segment of E1α-tp appear not to be critical for delivering protein into chloroplasts. Another possibility is that a similar motif in the downstream region of E1α-tp may compensate for the loss of the hydrophobic amino acid residues. However, the decrease in the size of the TP may not be tolerated.
Figure 2.
Multiple sequence motifs in the E1α-tp play a critical role in protein import into chloroplasts. A, Sequence of Ala substitution or deletion mutants. E1α-tp was divided into six 10-amino acid segments, T1 to T6, and the amino acid residues in these segments were substituted with Alas to produce E1α[T2A] to E1α[T6A]. In addition, the T1 segment was deleted to produce E1α[ΔT1]. B, Import experiments for Ala substitution and deletion mutants. Protoplasts were transformed with these E1α-tp mutants and protein import efficiency of the mutants was examined by western-blot analysis using an anti-GFP antibody (a) and quantified (b). The import efficiency is expressed as the percentage of the amount of the processed form divided by the total amount of expressed protein. Pre, Precursor forms; Pro, processed forms. C, Sequences of the second set of Ala substitution mutants. As with the second set of mutants, the restoration mutants were generated such that the five amino acid residues of the first or second half of each T segment were restored to the original sequence on the background of the Ala substitution mutants E1α[T2A] to E1α[T6A]. For the T1 segment, three Thr residues, three hydrophobic amino acid residues (F, P, and L), or one Lys residue were substituted with the same number of Alas to produce E1α[TTT/AAA], E1α[FPL/AAA], or E1α[K/A], respectively. D, Import experiments with the second set of Ala substitution mutants. Protoplasts were transformed with these E1α-tp mutants and protein import efficiency of the mutants was assessed by western-blot analysis using anti-GFP antibody. E, Quantification of targeting efficiency of E1α-tp, T4A, T4A+APPSS, and T4A+FLGST. The import efficiency is expressed as the percentage of the amount of the processed form divided by the total amount of expressed protein.
To narrow down the sequence motifs in the T3, T4, and T5 segments, we generated several restoration mutants, E1α[T3A+ENRLL], E1α[T3A+LPIRL], E1α[T4A+APPSS], E1α[T4A+FLGST], E1α[T5A+RSLSL], and E1α[T5A+RRLNH] (Fig. 2C). Restoration of either the ENRLL or the LPIRL sequence in the T3 segment completely reversed the defects of E1α[T3A] in protein import into chloroplasts (Fig. 2D, section b). In T4, the sequence motif APPSS, but not FLGST, restored the import efficiency of E1α[T4A] (Fig. 2, D [section c] and E). Finally, in T5, restoration of either the RSLSL or the RRLNH sequence was sufficient to fully recover the import efficiency of E1α[T5A] (Fig. 2D, section d). Together, these results indicate that ENRLL and LPIRL in T3, APPSS in T4, and RSLSL and RRLNH in T5 play critical roles in protein import into chloroplasts by E1α-tp.
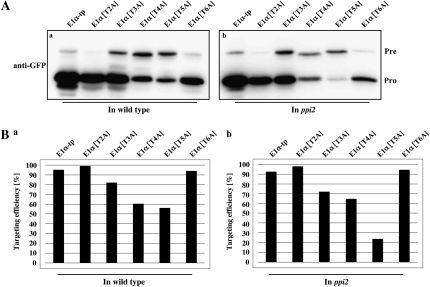
To test whether Ala substitution mutants of E1α-tp still conferred atToc159-independent import into chloroplasts, we expressed the GFP constructs with the Ala substitution mutant E1α-tp in ppi2 protoplasts (Fig. 3). Except for E1α[T5A], the other mutants were imported into chloroplasts at comparable levels in both wild-type and ppi2 mutant protoplasts. Surprisingly, in ppi2 protoplasts, E1α[T5A] showed a more severe defect in protein import than in the wild type (Fig. 3, A and B), indicating that the absence of a sequence motif in the T5 region of E1α-tp leads the mutant to adopt the atToc159-dependent pathway. This result raised the possibility that the T5 region of E1α TP may possess sequence motifs for the atToc132/atToc120-dependent pathway. More specifically, RSLSL or RRLNH motif in this region, both of which are critical for chloroplast import (Fig. 2, C and D), may function as atToc159-evading signal sequences.
Figure 3.
The T5 segment of E1α-tp contains a sequence motif for evading atToc159-dependent protein import into chloroplasts. A, Import experiments for Ala substitution mutants in wild-type (a) and ppi2 (b) protoplasts. Protoplasts were transformed with the E1α-tp mutant constructs and the protein import efficiency of these mutants was examined by western-blot analysis using an anti-GFP antibody. Pre, Precursor forms; Pro, processed forms. B, Quantification of targeting efficiencies of E1α-tp and its Ala substitution mutants. The import efficiency is expressed as the percentage of the amount of the processed form divided by the total amount of expressed protein.
Multiple Sequence Motifs in the RbcS-tp Are Involved in Determining Toc159 Dependence
To understand the mechanism of Toc159-dependent import into chloroplasts, we utilized RbcS-tp and E1α-tp, which confer Toc159 dependence and Toc159 independence in protein import into chloroplasts, respectively. We generated serial hybrid TPs of RbcS-tp and E1α-tp (Fig. 4A). In RbcS12/E1α-tp and E1α12/RbcS-tp, the N-terminal 12-amino acid fragments of E1α-tp and RbcS-tp were swapped. In the hybrid TPs E1α20/RbcS-tp, E1α30/RbcS-tp, E1α40-RbcS, E1α49-RbcS-tp, and E1α59/RbcS-tp, the 20-amino acid, 30-amino acid, 40-amino acid, 49-amino acid, and 59-amino acid N-terminal RbcS-tp fragments, respectively, were replaced with the corresponding E1α-tp N-terminal fragments. The import efficiency of these hybrid mutants was examined in wild-type protoplasts. All of the hybrid mutants, except E1α40/RbcS-tp, efficiently delivered GFP into chloroplasts in wild-type protoplasts when examined either by fluorescent microscopy or western-blot analysis using an anti-GFP antibody (Fig. 4, B and C). However, a majority of E1α40/RbcS-tp was present in the unprocessed or intermediate forms (Fig. 4C, section b). In a previous study, we demonstrated that RbcS-tp T4 contains the critical sequence motifs FP and RK (Lee et al., 2006). We restored these two critical sequence motifs, FP and RK, on the background of E1α40/RbcS-tp and transformed the resulting construct, E1α40/RbcS[FP, RK]-tp:GFP, into protoplasts. E1α40/RbcS[FP, RK]-tp:GFP was efficiently imported into chloroplasts, confirming the importance of the critical sequence motifs in RbcS-tp T4 (Fig. 4C, section c). Together, these results raise the possibility that sequence motifs located downstream of the RbcS-tp T4 segment cannot function together with sequence motifs in the N-terminal 40-amino acid segment of E1α-tp (Fig. 4C, section b). In addition, there were two different sizes of mature forms (Fig. 4C, section b). This difference may be due to alternative processing by stromal processing peptidase, which is caused by mutagenesis of RbcS TP.
To test whether these hybrid mutants underwent Toc159-dependent protein import into chloroplasts, they were transformed into ppi2 protoplasts either with or without T7:atTOC159 and their import efficiency was assessed by western-blot analysis using an anti-GFP antibody. Surprisingly, both the N-terminal 12-amino acid-swapped mutants, RbcS12/E1α-tp:GFP and E1α12/RbcS-tp:GFP, displayed Toc159-dependent import into chloroplasts (Fig. 4D, sections a–d), raising the possibility that Toc159 dependency is mediated by at least two independent regions in RbcS-tp. In support of this hypothesis, E1α20/RbcS-tp:GFP, E1α30/RbcS-tp:GFP, E1α40/RbcS-tp:GFP, and E1α49/RbcS-tp:GFP, but not E1α59/RbcS-tp, also displayed Toc159 dependency (Fig. 4D, sections e–l). These results further indicate that the Toc159 dependency of RbcS-tp is conferred by at least two independent regions, one in the N-terminal 12-amino acid fragment and the other in the region from T2 to T5 of RbcS-tp (Fig. 4D, sections a and c). In a previous study, multiple sequence motifs have been identified in T1 region and between the T2 and T5 regions of RbcS-tp (Lee et al., 2006, 2008).
To further investigate which segments between the T2 and T5 regions of RbcS TP are directly involved in determining Toc159-dependent protein import into chloroplasts, we generated new hybrid TP constructs, E1α[RbcST2]-tp, E1α[RbcST3]-tp, E1α[RbcST4]-tp, E1α[RbcST5]-tp, and E1α[RbcST6]-tp, in which the T2, T3, T4, T5, and T6 segments, respectively, of E1α-tp were replaced by the corresponding segments of RbcS-tp (Fig. 5A). These hybrid TPs were fused to GFP and introduced into wild-type and ppi2 protoplasts. The import efficiency of the hybrid TPs was determined by western-blot analysis using an anti-GFP antibody. The five hybrid TPs displayed different levels of import efficiency. E1α[RbcST2]-tp:GFP, E1α[RbcST3]-tp:GFP, and E1α[RbcST4]-tp:GFP were imported as efficiently as E1α-tp:GFP (Fig. 5, B and C). However, the import efficiency of E1α[RbcST5]-tp:GFP and E1α[RbcST6]-tp:GFP dropped to 50% and 23%, respectively, indicating that the T5 and T6 segments of RbcS-tp cannot adequately replace the T5 and T6 segments of E1α-tp as observed with E1α40/RbcS-tp (Fig. 5B). Among these five hybrid TPs, the import efficiency of E1α[RbcST5]-tp:GFP was further reduced to 32% in ppi2 protoplasts, indicating that the absence of atToc159 in ppi2 protoplasts caused a significant decrease in import efficiency (Fig. 5C). This result was also confirmed by complementation assay in ppi2 protoplasts (Fig. 5D). These results confirm that the T5 segment (amino acid positions 41–49) of RbcS-tp contains a sequence motif for Toc159-dependent protein import into chloroplasts. It was not possible to test T6 using this approach because T6 cannot support protein import into chloroplasts on the E1α-tp background (Fig. 5B).
Figure 5.
The RbcS-tp T5 segment confers Toc159 dependency to protein import of E1α-tp. A, Sequence of hybrid TPs of E1α-tp and RbcS-tp. The T2, T3, T4, T5, or T6 segment of E1α-tp was replaced with the corresponding segment from RbcS-tp. B, Western-blot analysis of import experiments. Protein extracts from wild-type or ppi2 protoplasts transformed with the indicated constructs were used for western-blot analysis using an anti-GFP antibody. The import experiments were performed three times. Pre, Precursor forms; Pro, processed forms. C, Quantification of import efficiency. The intensities of the bands shown in B were quantified as described in Figure 1F and the data shows the average import efficiency (n = 3; error bars, sd). D, Complementation analysis of E1α[RbcST5]-tp. E1α[RbcST5]-tp was cotransformed with R6 or T7:atTOC159 into ppi2 protoplasts and the protoplasts were analyzed by western-blot analysis with anti-GFP antibody.
Ser Residues in the N-Terminal 12-Amino Acid Segment of RbcS-tp Are Critical for Toc159-Dependent Protein Import into Chloroplasts
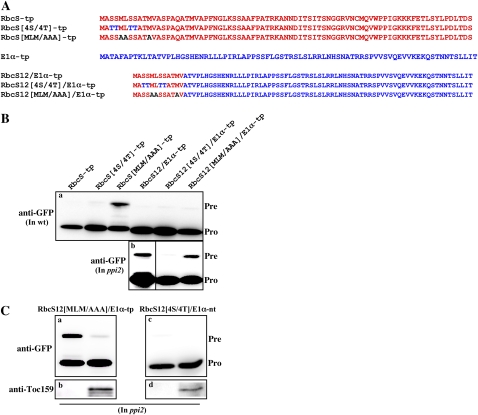
To further define the sequence requirements for Toc159 dependency in protein import into chloroplasts, we first compared the amino acid sequences of the RbcS-tp and E1α-tp N-terminal 12-amino acid segments. In previous studies, the hydrophobic amino acid residues in the first 10-amino acid segment of RbcS-tp have been shown to be critical for targeting to chloroplasts (Lee et al., 2006, 2008). In contrast, the hydrophobic amino acids in the first 10-amino acid segment of E1α did not play a critical role in protein import into chloroplasts (Fig. 2D). Based on these observations, we wanted to test whether the hydrophobic amino acids in the first 10-amino acid segment of RbcS-tp play any role in Toc159-dependent import into chloroplasts. The hydrophobic amino acid sequence motif MLM was substituted with Alas in the RbcS portion of the hybrid TP RbcS12/E1α-tp and fused to GFP (Fig. 6A). This construct, RbcS12[MLM/AAA]/E1α-tp:GFP, was introduced into wild-type protoplasts. As a control, RbcS[MLM/AAA]-tp:GFP was transformed into wild-type protoplasts. In wild-type protoplasts, RbcS12[MLM/AAA]/E1α-tp:GFP produced an almost undetectable level of the precursors, indicating that it was efficiently imported into chloroplasts (Fig. 6B, section a). However, as reported previously, RbcS[MLM/AAA]-tp:GFP produced a significant amount of precursors. These results suggest that the hydrophobic amino acids MLM are not as important in the hybrid TP RbcS12[MLM/AAA]/E1α-tp as they are in RbcS-tp (Fig. 6B, section a). However, in ppi2 protoplasts, RbcS12[MLM/AAA]-E1α-tp:GFP produced a significant amount of precursors, similar to RbcS12/E1α-tp:GFP, indicating that the Ala-substituted hybrid TP still contains the information for Toc159-dependent import into chloroplasts (Fig. 6B, section b).
Figure 6.
Ser residues in RbcS12 play a critical role in Toc159-dependent protein import into chloroplasts. A, Sequences of mutant TPs. In RbcS[4S/4T]-tp and RbcS[4S/4T]/E1α-tp, all the Ser residues in the N-terminal 12-amino acid region of RbcS-tp were replaced with Thrs. In RbcS[MLM/AAA]-tp and RbcS[MLM/AAA]/E1α-tp, the hydrophobic amino acids MLM in the first 10-amino acid segment of RbcS-tp were replaced with Alas. B and C, Import experiments with mutant TPs. Protoplasts from wild-type (wt; a) and ppi2 (b) plants were transformed with the indicated constructs together with empty vector or T7-atTOC159, and protein extracts were analyzed by western blotting using anti-GFP and anti-Toc159 antibodies. Pre, Precursor forms; Pro, processed forms.
To further confirm this, RbcS12[MLM/AAA]/E1α-tp:GFP was introduced into ppi2 protoplasts together with T7:atTOC159 and the import efficiency was determined by western-blot analysis using an anti-GFP antibody. When coexpressed with T7:atToc159, the amount of precursor was reduced to a barely detectable level, confirming that the reduced import efficiency of RbcS12[MLM/AAA]/E1α-tp:GFP into chloroplasts in ppi2 protoplasts is caused by the absence of atToc159 (Fig. 6C, section a). Expression of T7:atToc159 was confirmed by western-blot analysis using an anti-Toc159 antibody (Fig. 6C, section b).
To identify the sequence motif for Toc159-dependent protein import into chloroplasts, we more carefully examined the differences in the N-terminal RbcS-tp and E1α-tp 12-amino acid sequences. Interestingly, the N-terminal 12-amino acid segment of RbcS-tp is rich in Ser residues, whereas the corresponding E1α-tp segment is rich in Thr (Fig. 6A). To test whether Ser residues in the RbcS12 portion of the hybrid TP RbcS12/E1α-tp contribute to Toc159-dependent protein import into chloroplasts, all the Sers in the RbcS12 portion of RbcS12/E1α-tp were substituted with Thrs to produce RbcS12[4S/4T]/E1α-tp (Fig. 6A). This mutant TP was fused to GFP and the resulting construct, RbcS12[4S/4T]/E1α-tp:GFP, was introduced into wild-type protoplasts. The import efficiency of RbcS12[4S/4T]-E1α-tp:GFP was determined by western-blot analysis using an anti-GFP antibody. In wild-type protoplasts, RbcS12[4S/4T]/E1α-tp:GFP was efficiently imported into chloroplasts with an almost undetectable level of precursor, indicating that the substitution does not affect protein import into chloroplasts (Fig. 6B, section a). Similarly, RbcS12[4S/4T]/E1α-tp:GFP produced an almost undetectable level of precursor in ppi2 protoplasts, indicating that the absence of atToc159 in ppi2 protoplasts does not affect the protein import efficiency of RbcS12[4S/4T]/E1α-tp:GFP (Fig. 6B, section b). Consistent with this notion, coexpression of T7:atToc159 had no effect on the import efficiency of RbcS12[4S/4T]/E1α-tp:GFP (Fig. 6C, sections c and d). These results clearly demonstrate that in the N-terminal 12-amino acid segment of RbcS-tp, Ser residues play a critical role in Toc159-dependent protein import into chloroplasts.
The GM Domain of atToc159 Is Sufficient for Toc59-Dependent Protein Import into Chloroplasts
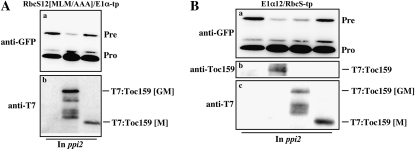
In a previous study, pea (Pisum sativum) RbcS-tp has been shown to directly bind to Toc159 (Becker et al., 2004). Both the N- and C-terminal domains of pea RbcS-tp showed direct interaction with Toc159 in vitro. Furthermore, the binding of Toc159 to nonphosphorylated C-terminal region of RbcS TP (pSSU) was stimulated in the presence of nonhydrolyzable 5-guanylyl-imidodiphosphate. By in vivo complementation experiments using ppi2 mutant, we determined the atToc159 domain essential for Toc159-dependent protein import into chloroplasts. In the case of RbcS-tp, Toc159-dependent protein import is conferred by at least two regions, one in the first 12 N-terminal amino acids and the other in the region between the T2 and T5 segments. First, we defined the domain of atToc159 that is involved in decoding information encoded by the RbcS-tp N-terminal 12-amino acid segment. The N-terminal 12-amino acid segment contains two pieces of functional sequence information, the hydrophobic amino acids MLM, and Ser residues. atToc159 can be divided into three main domains, the N-terminal acidic domain (A), the middle GTPase domain (G), and the C-terminal membrane domain (M; Bauer et al., 2000; Ivanova et al., 2004). Accordingly, we generated T7-tagged GM domain (T7:atTOC159-[GM]) and M domain (T7:atTOC159[M]) constructs and transformed them into ppi2 protoplasts together with RbcS12[MLM/AAA]/E1α-tp:GFP. Here, we used RbcS12[MLM/AAA]/E1α-tp:GFP, since Ser residues in the RbcS12 play a major role in Toc159-dependent protein import into chloroplasts (Fig. 6). Protein extracts were prepared from the transformed protoplasts and the import efficiency of RbcS12[MLM/AAA]-E1α-tp:GFP was determined by western-blot analysis using an anti-GFP antibody (Fig. 7A). The expression of T7:atToc159 was determined using an anti-T7 antibody. A coexpressed GM domain, but not an M domain alone, significantly reduced the amount of RbcS12[MLM/AAA]/E1α-tp:GFP precursor (Fig. 7A). These results strongly suggest that the GM domain of atToc159 is sufficient to support Toc159-dependent protein import that is mediated by Ser residues in the RbcS12. This is consistent with previous results showing that the A domain is not essential for complementation of ppi2 mutants (Lee et al., 2003; Agne et al., 2009).
Figure 7.
The GM domain of atToc159 is sufficient for Toc159-dependent protein import into chloroplasts. Full-length, GM domain, or M domain constructs of T7-tagged atTOC159 were transformed into ppi2 protoplasts together with RbcS12[MLM/AAA]E1α-tp:GFP (A) or E1α12/RbcS-tp:GFP (B), and import of these reporter proteins was examined by western-blot analysis using an anti-GFP antibody. Expression of T7:atToc159 protein was confirmed by western-blot analysis using anti-Toc159 and anti-T7 antibodies. Pre, Precursor forms; Pro, processed forms.
Next, we determined which domain of atToc159 is responsible for the Toc159 dependency mediated by the sequence motifs present in the region from the T2 to T5 segments of RbcS-tp. E1α12/RbcS-tp:GFP was cotransformed with T7:atTOC159, T7:atTOC159[GM], or T7:atTOC159[M], and the import efficiency was determined by western-blot analysis using anti-Toc159 or anti-GFP antibodies (Fig. 7B). Again, the full length and GM domain constructs, but not the M domain construct, of atToc159 showed significantly reduced levels of E1α12/RbcS-tp precursors, indicating that the GM domain is sufficient for Toc159-dependent protein import into chloroplasts (Fig. 7B).
DISCUSSION
Higher plant cells contain multiple isoforms of Toc159, which creates a situation in which protein import into plastids can occur by isoform-specific pathways such as the Toc159-dependent and Toc132/120-dependent pathways (Ivanova et al., 2004; Kubis et al., 2004). In this study, we investigated the molecular mechanisms of Toc159-dependent protein import into chloroplasts using RbcS-tp as a model TP and using a transient expression approach in protoplasts. Consistent with previous results (Ivanova et al., 2004; Smith et al., 2004), we demonstrated that RbcS-tp and E1α-tp confer Toc159-dependent and Toc159-independent import of proteins into chloroplasts in leaf tissue protoplasts, respectively (Fig. 1).
Next, we identified functional sequence motifs in the TP of E1α. Unlike RbcS TP, the N-terminal hydrophobic residues were not important for chloroplast targeting, which is also true of the TPs of Cab, BCCP, and GLU2 (Lee et al., 2008). Interestingly, E1α[T5A]-tp:GFP showed more severe defect in chloroplast targeting in ppi2 mutant protoplasts than in wild-type protoplasts (Fig. 3). Moreover, E1α[RbcST5]-tp:GFP, in which T5 segment of E1α-tp was replaced with the corresponding segment of RbcS-tp, was atToc159-dependent for efficient chloroplast import (Fig. 5). These results suggest that T5 segment of each TP is involved in determining their own targeting pathways, and detailed functional analysis of these regions will be necessary to elucidate multiple targeting pathways to chloroplasts further. One possibility is that T5 segment of E1α is required for interaction with atToc132/atToc120, but not with atToc159.
Hybrid TPs generated using RbcS-tp and E1α-tp revealed that Toc159-dependent protein import of RbcS-tp into chloroplasts is mediated independently by at least two different domains, one in the N-terminal 12-amino acid region and the other in the C-terminal region ranging from the T2 to T5 segments of RbcS-tp (Fig. 4).
We then carried out detailed analysis of the important sequence features of the N-terminal 12-amino acid segment of RbcS-tp (Fig. 6). In a previous study, the N-terminal region was shown to play a role in targeting to the chloroplasts (Lee et al., 2006, 2008). Specifically, the hydrophobic amino acid residues in the N-terminal region are critical for the import of protein into chloroplasts. Consistent with this, bioinformatics analysis of the transit peptidome revealed the presence of hydrophobic amino acid residues in a majority of TPs (Bruce, 2001; Zhang and Glaser, 2002; Patron and Waller, 2007; Lee et al., 2008). However, the most critical amino acids enabling Toc159-dependent protein import into chloroplasts were the Ser residues in the N-terminal 12-amino acid segment of RbcS. Surprisingly, substitution of these Sers with Thrs in the hybrid TP RbcS12/E1α-tp abolished Toc159-dependent protein import into chloroplasts, thus clearly demonstrating that Ser residues in the N-terminal 12-amino acid fragment of RbcS-tp are a signal for Toc159-dependent protein import into chloroplasts (Fig. 6). Previously, it had been noted that hydroxylated amino acids are frequently observed in the TPs, although the reason for this has not been understood (Zhang and Glaser, 2002; Patron and Waller, 2007). This is the first clearly defined function of Ser residues in the TP during protein import into chloroplasts. However, it is not currently known what factors differentiate between the Ser and Thr residues in the TP.
Toc159-dependent protein import into chloroplasts was also mediated by the region ranging from the T2 to the T5 segment of RbcS-tp (Fig. 4). Among these regions, only the T5 segment conferred Toc159 dependency to E1α-tp when we examined the Toc159 dependency using the domain-swapping mutants (Fig. 5). However, we do not exclude the possibility that other sequence motifs between T2 and T5 regions are also involved in Toc159-dependent protein import into chloroplasts because Toc159 dependency was gradually reduced as the portion of E1α-tp in the hybrid TPs was increased (Fig. 4). Previously, it had been shown that multiple sequence motifs are present in this region of RbcS-tp and that the C-terminal region of RbcS-tp binds to atToc159 in vitro (Becker et al., 2004; Smith et al., 2004; Lee et al., 2006). One possibility is that these multiple sequence motifs may include Toc159 binding motifs that play a role in Toc159-dependent protein import into chloroplasts. Consistent with this hypothesis, the GM domain, but not the M domain alone, was able to compensate for ppi2 mutation. However, we cannot exclude the possibility that a cytosolic factor may play a role at some point before atToc59 in the Toc159-dependent protein import pathway. Cytosolic Hsp70 and Hsp90 have previously been implicated in protein import into chloroplasts (Jackson-Constan et al., 2001; Zhang and Glaser, 2002; Qbadou et al., 2006). Indeed, sequence analysis of TPs revealed the presence of multiple binding sites for Hsp70 (Zhang and Glaser, 2002). In addition, it has been proposed that 14-3-3 forms a guidance complex for TPs (May and Soll, 2000). Thus, one possible scenario is that a cytosolic factor may first bind to Toc159-dependent TPs and subsequently deliver these peptides to atToc159 in Arabidopsis (Arabidopsis thaliana). Thus, the cytosolic factor and Toc159 would constitute the Toc159-dependent pathway. In fact, precursors bound to cytosolic Hsp90 are specifically delivered to atToc64 (Qbadou et al., 2006). Similar to protein import into mitochondria in animal cells, precursors bound to Hsp90/Hsp70 are specifically delivered to Tom70, but not to Tom20 (Young et al., 2003; Rehling et al., 2004). However, it currently remains largely unknown whether any protein factor is involved in import into chloroplasts before Toc159. Further studies will be necessary for a full understanding of the Toc159-dependent and Toc159-independent import pathways.
MATERIALS AND METHODS
Growth of Plants
Arabidopsis (Arabidopsis thaliana; Columbia ecotype) was grown in soil at 20°C to 25°C in a greenhouse with a 16-h/8-h light/dark cycle, or on Murashige and Skoog plates in a growth chamber at 22°C. Leaf tissues were harvested from 2-week-old plants and used immediately for protoplast isolation.
PCR-Based Mutagenesis and Plasmid Construction
Ala substitution mutant E1α-tp:GFP constructs were generated using standard molecular cloning techniques, as described previously (Lee et al., 2006). The swapping mutants generated from RbcS-tp:GFP and E1α-tp:GFP were also constructed using PCR. For example, for construction of RbcS12-E1α-tp:GFP, a primer (Top) consisting of a 20 mer from the RbcS12 3′ region, a 20 mer from the 5′ region of E1α-tp:GFP, and its reverse complement primer (Bottom) were synthesized. Using E1α-tp:GFP as a template, PCR was performed using the Top and nos-t primers. For RbcS-tp:GFP, PCR was performed using the cauliflower mosaic virus and Bottom primers. Each PCR product was extracted from an agarose gel and, using both PCR products as templates, a second PCR was performed using the cauliflower mosaic virus and nos-t primers. The PCR product was then digested with XbaI and SacI, and ligated into a pUC-based expression vector. The primer sequences used to prepare the constructs are shown in Supplemental Table S1.
Transient Expression in Protoplasts
The plasmids used in protoplast transformation were purified using Qiagen columns (Basel), according to the manufacturer's protocol. DNA was introduced into Arabidopsis protoplasts prepared from leaf tissues by polyethylene glycol-mediated transformation (Jin et al., 2001; Kim et al., 2001).
Images were obtained using a cooled CCD camera and a Zeiss Axioplan fluorescence microscope. The filter sets used were XF116 (exciter, 474AF20; dichroic, 500DRLP; emitter, 510AF23) and XF33/E (exciter, 535DF35; dichroic, 570DRLP; emitter, 605DF50; Omega, Inc.) for GFP/fluorescein isothiocyanate and red fluorescent protein (RFP)/tetramethylrhodamine B isothiocyanate, respectively. The data was processed using Adobe Photoshop software and the images were rendered in pseudocolor.
Western-Blot Analysis and Signal Intensity Quantification
Western-blot analysis was performed using protein extracts from the transformed protoplasts, as described previously (Jin et al., 2001; Kim et al., 2001). Briefly, protoplasts were lysed by brief sonication in lysis buffer (10 mm Tris-HCl, pH 7.5, 150 mm NaCl, 2.0 mm EDTA, 0.2 mm phenylmethylsulfonyl fluoride) and subjected to centrifugation at 10,000g at 4°C for 10 min to remove debris. Proteins in the supernatants were separated by SDS-PAGE. Immunoreactive proteins were visualized using enhanced chemiluminescence (ECL kit, Amersham Pharmacia Biotech), and images were obtained using a LAS3000 image capture system (FUJIFILM). The immunoblots were quantified by measuring the intensity of the protein bands with LAS3000 software. Immunoblot images were obtained at different exposure times. Only images whose band intensities were within the linear range between the intensity increase and exposure time were selected.
Chloroplast Purification
To isolate chloroplasts, protoplasts were gently lysed in ice-cold HMS buffer (330 mm sorbitol, 50 mm HEPES/KOH, pH 7.6, 3 mm MgCl2). The lysed mixtures were overlaid onto two silica sol gradients (Percoll; 2 mL of 80% [v/v] Percoll in 330 mm sorbitol, 50 mm HEPES/KOH, pH 7.6; 4 mL of 40% [v/v] Percoll in 330 mm sorbitol, 50 mm HEPES/KOH, pH 7.6), and centrifuged for 5 min at 3,000g. After centrifugation, intact chloroplasts were carefully collected, transferred to 10 mL of HMS buffer, and pelleted by centrifugation for 5 min at 3,000g. Chloroplast extracts were prepared from the isolated chloroplasts and used for immunoblotting with an anti-GFP antibody.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AAG40356 (RbcS) and NP_171617 (E1α).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Sequences of primers used in this study.
Supplementary Material
Acknowledgments
Anti-atToc159 antibody was obtained from Felix Kessler (Université de Neuchâtel, Switzerland). The authors would like to thank Young Hee Shin, Kyung Hwa Jung, and Sang Kook Lee (POSTECH, Korea) for growing plants and assistance.
This work was supported by grants from the Systems Bio-Dynamics-National Core Research Center (grant no. R15–2004–033–05002–0), Crop Functional Genomics Center and the Agricultural Research and Planning Center (grant no. 609004–05–1–SB210), Crop Functional Genomics Center (grant no. 2009K001190), and Biogreen 21 (grant no. 20070401–034–026–009–04–00) Rural Development Administration.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Inhwan Hwang (ihhwang@postech.ac.kr).
The online version of this article contains Web-only data.
Open access articles can be viewed online without a subscription.
References
- Agne B, Infanger S, Wang F, Hofstetter V, Rahim G, Martin M, Lee DW, Hwang I, Schnell D, Kessler F (2009) A toc159 import receptor mutant, defective in hydrolysis of GTP, supports preprotein import into chloroplasts. J Biol Chem 284 8670–8679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J, Chen K, Hiltbunner A, Wehrli E, Eugster M, Schnell D, Kessler F (2000) The major protein import receptor of plastids is essential for chloroplast biogenesis. Nature 403 203–207 [DOI] [PubMed] [Google Scholar]
- Becker T, Jelic M, Vojta A, Radunz A, Soll J, Schleiff E (2004) Preprotein recognition by the Toc complex. EMBO J 23 520–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce BD (2001) The paradox of plastid transit peptides: conservation of function despite divergence in primary structure. Biochim Biophys Acta 1541 2–21 [DOI] [PubMed] [Google Scholar]
- Chen KY, Li HM (2007) Precursor binding to an 880-kDa Toc complex as an early step during active import of protein into chloroplasts. Plant J 49 149–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelsson O, Nielsen H, Brunak S, von Heijne G (2000) Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol 300 1005–1016 [DOI] [PubMed] [Google Scholar]
- Ivanova Y, Smith MD, Chen K, Schnell DJ (2004) Members of the Toc159 import receptor family represent distinct pathways for protein targeting to plastids. Mol Biol Cell 15 3379–3392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson-Constan D, Akita M, Keegstra K (2001) Molecular chaperones involved in chloroplast protein import. Biochim Biophys Acta 1541 102–113 [DOI] [PubMed] [Google Scholar]
- Jarvis P (2008) Targeting of nucleus-encoded proteins to chloroplasts in plants. New Phytol 179 257–285 [DOI] [PubMed] [Google Scholar]
- Jin JB, Kim YA, Kim SJ, Lee SH, Kim DH, Cheong GW, Hwang I (2001) A new dynamin-like protein, ADL6, is involved in trafficking from the trans-Golgi network to the central vacuole in Arabidopsis. Plant Cell 13 2175–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler F, Schnell DJ (2006) The function and diversity of plastid protein import pathways: a multilane GTPase highway into plastids. Traffic 7 248–257 [DOI] [PubMed] [Google Scholar]
- Kim DH, Eu YJ, Yoo CM, Kim YW, Pih KT, Jin JB, Kim SJ, Stenmark H, Hwang I (2001) Trafficking of phosphatidylinositol 3-phosphate from the trans-Golgi network to the lumen of the central vacuole in plant cells. Plant Cell 13 287–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubis S, Patel R, Combe J, Bédard J, Kovacheva S, Lilley K, Biehl A, Leister D, Ríos G, Koncz C, et al (2004) Functional specialization amongst the Arabidopsis Toc159 family of chloroplast protein import receptors. Plant Cell 16 2059–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DW, Kim JK, Lee S, Choi S, Kim S, Hwang I (2008) Arabidopsis nuclear-encoded plastid transit peptides contain multiple sequence subgroups with distinctive chloroplast-targeting sequence motifs. Plant Cell 20 1603–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DW, Lee S, Lee GJ, Lee KH, Kim S, Cheong GW, Hwang I (2006) Functional characterization of sequence motifs in the transit peptide of Arabidopsis small subunit of rubisco. Plant Physiol 140 466–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Kim DH, Lee SW, Kim ZH, Hwang I (2002) In vivo import experiments in protoplasts reveal the importance of the overall context but not specific amino acid residues of the transit peptide during import into chloroplasts. Mol Cells 14 388–397 [PubMed] [Google Scholar]
- Lee KH, Kim SJ, Lee YJ, Jin JB, Hwang I (2003) The M domain of atToc159 plays an essential role in the import of proteins into chloroplasts and chloroplast biogenesis. J Biol Chem 278 36794–36805 [DOI] [PubMed] [Google Scholar]
- May T, Soll J (2000) 14-3-3 proteins form a guidance complex with chloroplast precursor proteins in plants. Plant Cell 12 53–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miras S, Salvi D, Ferro M, Grunwald D, Garin J, Joyard J, Rolland N (2002) Non-canonical transit peptide for import into the chloroplast. J Biol Chem 277 47770–47778 [DOI] [PubMed] [Google Scholar]
- Miras S, Salvi D, Piette L, Seigneurin-Berny D, Grunwald D, Reinbothe C, Joyard J, Reinbothe S, Rolland N (2007) Toc159- and Toc75-independent import of a transit sequence-less precursor into the inner envelope of chloroplasts. J Biol Chem 282 29482–29492 [DOI] [PubMed] [Google Scholar]
- Nada A, Soll J (2004) Inner envelope protein 32 is imported into chloroplasts by a novel pathway. J Cell Sci 117 3975–3982 [DOI] [PubMed] [Google Scholar]
- Nakrieko KA, Mould RM, Smith AG (2004) Fidelity of targeting to chloroplasts is not affected by removal of the phosphorylation site from the transit peptide. Eur J Biochem 271 509–516 [DOI] [PubMed] [Google Scholar]
- Patron NJ, Waller RF (2007) Transit peptide diversity and divergence: a global analysis of plastid targeting signals. Bioessays 29 1048–1058 [DOI] [PubMed] [Google Scholar]
- Pilon M, Wienk H, Sips W, de Swaaf M, Talboom I, van't Hof R, de Korte-Kool G, Demel R, Weisbeek P, de Kruijff B (1995) Functional domains of the ferredoxin transit sequence involved in chloroplast import. J Biol Chem 270 3882–3893 [DOI] [PubMed] [Google Scholar]
- Qbadou S, Becker T, Mirus O, Tews I, Soll J, Schleiff E (2006) The molecular chaperone Hsp90 delivers precursor proteins to the chloroplast import receptor Toc64. EMBO J 25 1836–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehling P, Brandner K, Pfanner N (2004) Mitochondrial import and the twin-pore translocase. Nat Rev Mol Cell Biol 5 519–530 [DOI] [PubMed] [Google Scholar]
- Rensink WA, Schnell DJ, Weisbeek PJ (2000) The transit sequence of ferredoxin contains different domains for translocation across the outer and inner membrane of the chloroplast envelope. J Biol Chem 275 10265–10271 [DOI] [PubMed] [Google Scholar]
- Richter S, Lamppa GK (1999) Stromal processing peptidase binds transit peptides and initiates their ATP-dependent turnover in chloroplasts. J Cell Biol 147 33–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MD, Rounds CM, Wang F, Chen K, Afitlhile M, Schnell DJ (2004) AtToc159 is a selective transit peptide receptor for the import of nucleus-encoded chloroplast proteins. J Cell Biol 165 323–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G, Steppuhn J, Herrmann RG (1989) Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem 180 535–545 [DOI] [PubMed] [Google Scholar]
- Young JC, Hoogenraad NJ, Hartl FU (2003) Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell 112 41–50 [DOI] [PubMed] [Google Scholar]
- Zhang XP, Glaser E (2002) Interaction of plant mitochondrial and chloroplast signal peptides with the Hsp70 molecular chaperone. Trends Plant Sci 7 14–21 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.