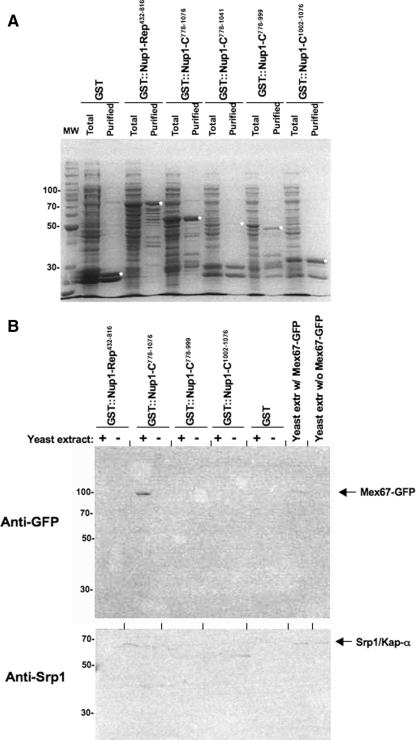
Figure 3.
Students did not identify a subdomain of GST-Nup1-C that associates with Mex67-GFP. (A) Students expressed GST-Nup1 fusion proteins in E. coli and purified the recombinant GST fusion proteins using glutathione-Sepharose beads. For each sample, a lane containing total bacterial protein extract (Total) and a lane containing purified GST-Nup1 protein (Purified) was separated by SDS-PAGE and stained with Coomassie Blue. Full-length purified proteins are indicated with a white asterisk (*). (B) Affinity-purified GST-Nups bound to beads were incubated with yeast extracts containing Mex67-GFP (+) or with a mock solution lacking yeast proteins (−). Students used Western blotting with anti-GFP (top) and anti-Srp1 (bottom) antibodies to detect the presence or absence of Mex67-GFP and Srp1/Kap-α in each sample. Total yeast extracts containing or lacking Mex67-GFP were run as controls for detection of the GFP epitope. Bands representing Mex67-GFP and Srp1/Kap-α are indicated with the labeled arrows. Molecular weight standards (in kilodaltons) are depicted to the left of each gel.