Abstract
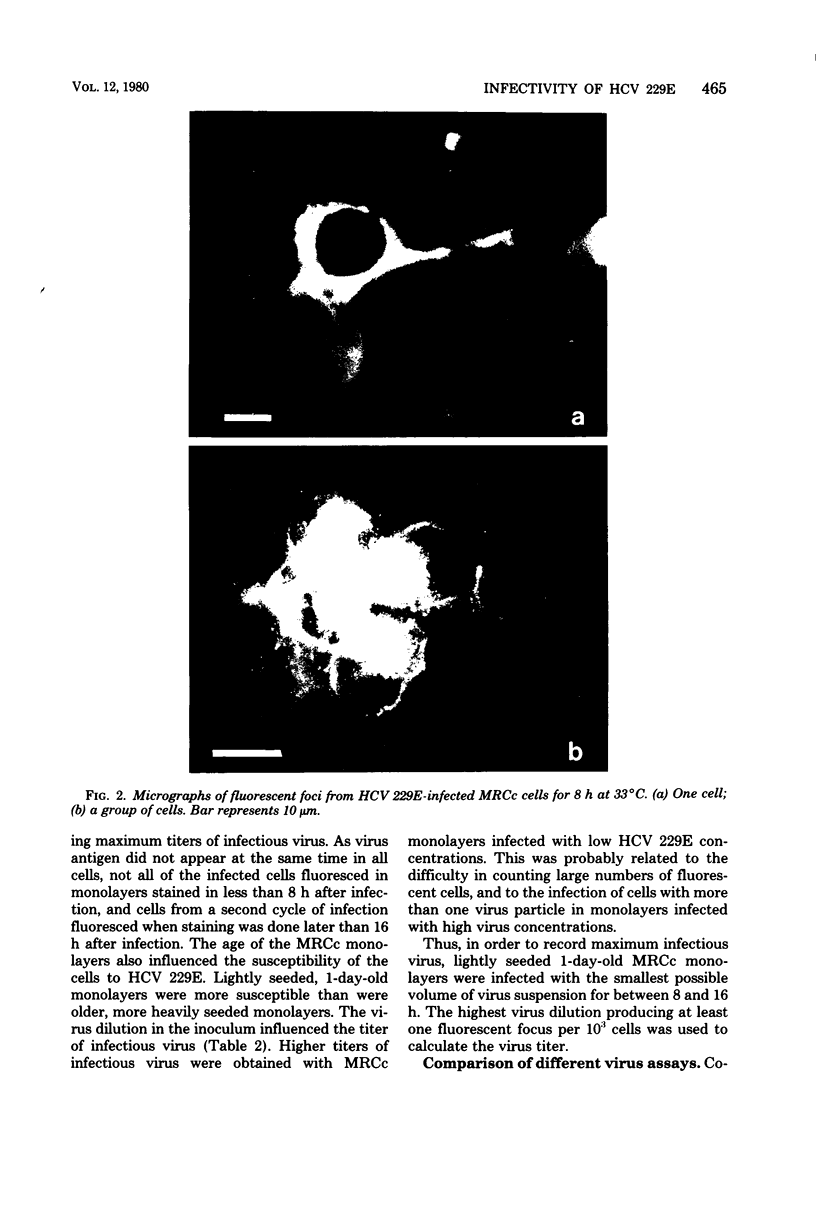
The replication of human coronavirus strain 229E was observed by using indirect immunofluorescence in infected monolayers of MRC continuous cells. By 8 h after infection, bright cytoplasmic fluorescence was detected in cells infected with human coronavirus 229E. Discrete foci of infection were observed from 8 to 16 h after infection in cells infected with high dilutions of human coronavirus 229E; each fluorescent focus corresponded to a single virus infection. A fluorescent focus assay is described, using indirect immunofluorescence, which is more sensitive than the established techniques of tube titration and plaque assay. Particle/infectivity ratios for unpurified and purified virus preparations revealed a considerable drop in infectivity on purification.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker W. B., McIntosh K., Dees J. H., Chanock R. M. Morphogenesis of avian infectious bronchitis virus and a related human virus (strain 229E). J Virol. 1967 Oct;1(5):1019–1027. doi: 10.1128/jvi.1.5.1019-1027.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradburne A. F., Tyrrell D. A. The propagation of "coronaviruses" in tissue-culture. Arch Gesamte Virusforsch. 1969;28(2):133–150. doi: 10.1007/BF01249379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucknall R. A., King L. M., Kapikian A. Z., Chanock R. M. Studies with human coronaviruses. II. Some properties of strains 229E and OC43. Proc Soc Exp Biol Med. 1972 Mar;139(3):722–727. doi: 10.3181/00379727-139-36224. [DOI] [PubMed] [Google Scholar]
- Davies H. A., Macnaughton M. R. Comparison of the morphology of three coronaviruses. Arch Virol. 1979;59(1-2):25–33. doi: 10.1007/BF01317891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamre D., Kindig D. A., Mann J. Growth and intracellular development of a new respiratory virus. J Virol. 1967 Aug;1(4):810–816. doi: 10.1128/jvi.1.4.810-816.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamre D., Procknow J. J. A new virus isolated from the human respiratory tract. Proc Soc Exp Biol Med. 1966 Jan;121(1):190–193. doi: 10.3181/00379727-121-30734. [DOI] [PubMed] [Google Scholar]
- Hierholzer J. C. Purification and biophysical properties of human coronavirus 229E. Virology. 1976 Nov;75(1):155–165. doi: 10.1016/0042-6822(76)90014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D. A., Johnson-Lussenburg C. M. Isolation and morphology of the internal component of human coronavirus, strain 229E. Intervirology. 1975;6(4-5):197–206. doi: 10.1159/000149474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnaughton M. R., Davies H. A. Two particle types of avian infectious bronchitis virus. J Gen Virol. 1980 Apr;47(2):365–372. doi: 10.1099/0022-1317-47-2-365. [DOI] [PubMed] [Google Scholar]
- Macnaughton M. R., Madge M. H. The genome of human coronavirus strain 229E. J Gen Virol. 1978 Jun;39(3):497–504. doi: 10.1099/0022-1317-39-3-497. [DOI] [PubMed] [Google Scholar]
- Monto A. S., Rhodes L. M. Detection of coronavirus infection of man by immunofluorescence. Proc Soc Exp Biol Med. 1977 Jun;155(2):143–148. doi: 10.3181/00379727-155-39761. [DOI] [PubMed] [Google Scholar]
- Schmidt O. W., Cooney M. K., Kenny G. E. Plaque assay and improved yield of human coronaviruses in a human rhabdomyosarcoma cell line. J Clin Microbiol. 1979 Jun;9(6):722–728. doi: 10.1128/jcm.9.6.722-728.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell D. A., Alexander D. J., Almeida J. D., Cunningham C. H., Easterday B. C., Garwes D. J., Hierholzer J. C., Kapikian A., Macnaughton M. R., McIntosh K. Coronaviridae: second report. Intervirology. 1978;10(6):321–328. doi: 10.1159/000148996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON D. H., RUSSELL W. C., WILDY P. Electron microscopic particle counts on herpes virus using the phosphotungstate negative staining technique. Virology. 1963 Mar;19:250–260. doi: 10.1016/0042-6822(63)90062-x. [DOI] [PubMed] [Google Scholar]