Table 5. Selective Synthesis of 5.
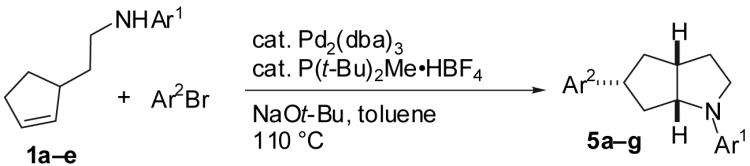 | ||||
|---|---|---|---|---|
| Entry | Substrate(b) | Crude Ratio 2:3:4:5 | Product | Isolated Yield 5 |
| 1 | 1a | 0:0:0:100 | 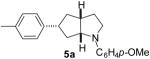 |
74 % |
| 2 | 1a | 0:0:0:100 | 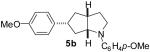 |
75 % |
| 3 | 1b | 0:15:0:85 | 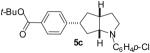 |
71 % |
| 4 | 1d | 0:10:0:90 | 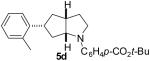 |
69 % |
| 5 | 1c | 5:10:10:75 | 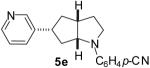 |
67 %(c) |
| 6 | 1e | 0:10:0:90 | 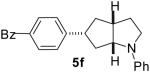 |
85 % |
| 7 | 1e | 0:0:0:100 | 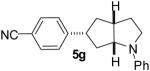 |
76 % |
Conditions: 1.0 equiv amine, 1.4 equiv Ar2Br, 1.2 equiv NaOt-Bu, 1 mol % Pd2(dba)3, 2 mol % P(t-Bu)2Me·HBF4, toluene (0.25 M), 110 °C.
1a : Ar = p-(MeO)C6H4; 1b: Ar = p-(Cl)C6H4; 1c: Ar = p-(NC)C6H4; 1d: Ar =p-(t-BuO2C)C6H4; 1e Ar = Ph.
This material contained 7 % of isomer 3 as an inseparable impurity.
