Abstract
Since their recruitment in the oral cavity, approximately 450 million years ago, teeth have been subjected to strong selective constraints due to the crucial role that they play in species survival. It is therefore quite surprising that the ability to develop functional teeth has subsequently been lost several times, independently, in various lineages. In this review, we concentrate our attention on tetrapods, the only vertebrate lineage in which several clades lack functional teeth from birth to adulthood. Indeed, in other lineages, teeth can be absent in adults but be functionally present in larvae and juveniles, can be absent in the oral cavity but exist in the pharyngeal region, or can develop on the upper jaw but be absent on the lower jaw. Here, we analyse the current data on toothless (edentate) tetrapod taxa, including information available on enamel-less species. Firstly, we provide an analysis of the dispersed and fragmentary morphological data published on the various living taxa concerned (and their extinct relatives) with the aim of tracing the origin of tooth or enamel loss, i.e. toads in Lissamphibia, turtles and birds in Sauropsida, and baleen whales, pangolins, anteaters, sloths, armadillos and aardvark in Mammalia. Secondly, we present current hypotheses on the genetic basis of tooth loss in the chicken and thirdly, we try to answer the question of how these taxa have survived tooth loss given the crucial importance of this tool. The loss of teeth (or only enamel) in all of these taxa was not lethal because it was always preceded in evolution by the pre-adaptation of a secondary tool (beak, baleens, elongated adhesive tongues or hypselodonty) useful for improving efficiency in food uptake. The positive selection of such secondary tools would have led to relaxed functional constraints on teeth and would have later compensated for the loss of teeth. These hypotheses raise numerous questions that will hopefully be answered in the near future.
Keywords: birds, enamel, evolution, mammals, toads, tooth loss, turtles
Introduction
Teeth originated in stem gnathostomes, approximately 450 million years ago (Ma) (Reif, 1982; Donoghue, 2002; Donoghue & Sansom, 2002; Donoghue et al. 2006). Two theories have been proposed for the evolution of teeth. Firstly, that teeth derived from skin denticles/odontodes that moved from the outside of the mouth in and, alternatively, that teeth derived from pharyngeal denticle whorls and moved up into the mouth (Smith, 2003; see review by Huysseune et al. 2009). Either way the newly acquired oral structures played an important role in concert with jaws, allowing for a predatory lifestyle, and their development would have been selectively constrained. During the further evolution of gnathostomes, these original ‘teeth’ diversified, changing their location, shape and ornamentation, size, mode of attachment and number of generations, such diversification permitting adaptation to a variety of diets (Huysseune & Sire, 1998). Although exhibiting morphological differences in various vertebrate taxa, teeth have mostly conserved their original structure, i.e. a pulp cavity surrounded by a dentine crown generally covered by enamel or enameloid, two hard protective tissues. Such a long-lasting conservation of tooth organization and structure is related to strong selective constraints that result from the important role played by these organs. This also explains why teeth are still present in all living gnathostome lineages [chondrichthyans (cartilaginous fish), actinopterygians (ray-finned fish) and sarcopterygians (coelacanth, lungfish and tetrapods: lissamphibians, reptiles and mammals)]. However, although teeth and their protective tissues (enamel/enameloid) seem to be crucial tools for animal survival, they have been lost in several vertebrate taxa. The three lineages of tetrapods mentioned above include several toothless and enamel-less taxa. This review focuses on these taxa and provides data on their evolution and adaptation. We have considered only the tetrapods because, to our knowledge, there are no examples of edentate species in chondrichthyans, coelacanths, lungfish and actinopterygian fish. In the latter lineage, however, we know of numerous species that either lack teeth in the oral cavity but retain them in the pharyngeal region only (e.g. all cypriniforms, such as carp, roach and zebrafish) or possess functional teeth in larvae and juveniles that are shed and not replaced in further stages (e.g. sturgeons, armoured catfish) (Sire et al. 2002; see also Huysseune et al. 2009). We will not describe tetrapod species that have lost teeth on one jaw but retain them in the other, such as some odontocetes (e.g. sperm whales) and most anuran amphibians (e.g. clawed toads). In mammals, however, we will comment on the platypus (monotremes) as this species possesses teeth in the embryos and juveniles but loses them in the adult.
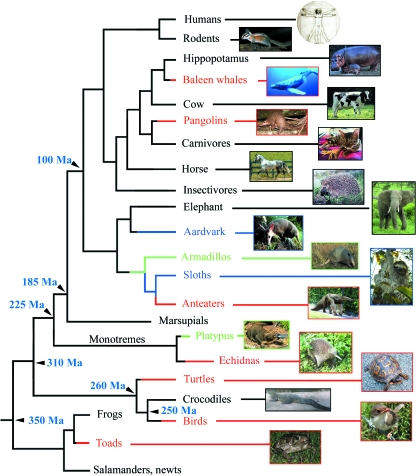
The location of all living edentate taxa in the tetrapod phylogeny clearly indicates that, during evolution, (i) the ability to form teeth was lost independently in seven lineages and (ii) enamel disappeared in two unrelated lineages (Fig. 1). This suggests that teeth and/or enamel could be ‘easily’ lost; however, we will see that species survival after tooth or enamel loss strongly depends on the recruitment of various pre-existing morphological adaptations that were secondarily retained as more efficient mechanisms for food processing.
Fig. 1.
Simplified tetrapodan phylogeny with indication of toothless lineages (red lines), enamel-less lineages (blue lines) and lineages with enamel reduction and tooth reduction (green lines). Tetrapodan relationships after Murphy et al. (2001) and Hedges (2002).
In humans, most cases of tooth loss (agenesis) are related to syndromes that result from mutations in various genes (e.g. ectodysplasia, Mikkola & Thesleff, 2003; see Caton & Tucker, 2009; De Coster et al. 2009). Non-syndromic tooth loss in humans has so far been linked to three genes (Pax9, Msx1 and Axin2) that lead to hypodontia (loss of a few teeth) and oligodontia (loss of at least six teeth) (Matalova et al. 2008). To our knowledge, there are no reports in the literature on completely edentate patients in which no other phenotype was identified and which are not related to a syndrome.
Here, we review the current knowledge of edentate and enamel-less tetrapods, and we present scenarios that could explain how the ability to form teeth was lost and why these taxa survived tooth loss. These topics are covered in four sections. The first section is devoted to tooth loss in chicken, as this is the only edentate species in which developmental mechanisms underlying the loss of teeth have been studied. The second section is a review of the current knowledge of tooth loss in the other edentate lineages. For each lineage, data are compiled on tooth loss during evolution and ontogeny. The situation in the adult is presented and the specialized morphological adaptations that were selected during evolution as improving food uptake when teeth were lost are pointed out. The third section concerns enamel loss in two mammalian lineages [Xenarthra (sloths and armadillos) and Tubulidentata (Afrotheria: aardvark)] with particular attention to the nine-banded armadillo. The fourth section provides information on the platypus, a monotreme species in which teeth are lacking in adults but are present in embryos and juveniles. When available, we have included palaeontological data that can support a tentative datation of tooth loss or enamel loss in these taxa and the relationships of these taxa. These findings are then briefly discussed in the conclusion.
Tooth loss in birds and the famous hen's teeth
Fossil and living birds constitute the Avialae (sensu Gauthier, 1986) (Fig. 2). The living species (around 10 000 species in 26 orders) are divided into the Paleognathae and the Neognathae (Sibley & Ahlquist, 1990). Both form the clade Neornithes. All of them are toothless.
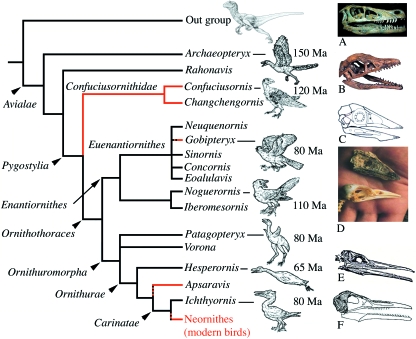
Fig. 2.
Simplified phylogeny of Avialae (after Chiappe, 2002). Lineages with toothless taxa are in red. (A) Skull of Velociraptor. (B) Skull of Archaeopteryx lithographica. (C) Skull of Confuciusornis sanctus. (D) Comparison of the skulls ofC. sanctus and a pigeon (bottom). (E) Skull of Hesperornis regalis (after Marsh, 1880). (F) Ichthyornis dispar (after Marsh, 1883).
What the fossil record tells us
It is generally believed that modern birds (Neornithes) derive from theropod dinosaurs (e.g. Ostrom, 1976), although a close relationship between birds and crocodilians has also been proposed on the basis, amongst other characters, of similarities of tooth ornamentation (Whetstone & Martin, 1979; Martin et al. 1980). The last common ancestor of Avialae was equipped with clearly functional teeth. The oldest widely accepted Avialae is the well-known Archaeopteryx lithographica, which lived some 150 Ma and possessed teeth. More than 70 avialan genera are known from the Mesozoic (140–120 Ma; Chiappe & Dyke, 2002), which allows a phylogenetic tree to be drawn based on anatomical characters (Fig. 2). The most recent toothed Avialae in fossil records, the ornithurine birds Hesperornis regalis and Ichthyornis dispar, are known from the late Cretaceous (93–65 Ma; Marsh, 1872; Gregory, 1952). H. regalis was a swimming bird. It had a long beak with a rhamphotheca covering the pre-maxilla region only and was provided with small but effective conical teeth set firmly in the jaw (Gingerich, 1975). The teeth of the upper jaws were few in number and set in the back part, whereas those of the mandibles formed a complete series (Fig. 2). H. regalis therefore had half a beak and teeth. To date, I. dispar is the closest Avialae to the common ancestor of Neornithes (Clarke, 2004). The teeth of I. dispar are set in a groove as in H. regalis, and they are broad and flattened with highly expanded roots (Martin & Stewart, 1977). I. dispar was a powerful flighting bird and did not differ notably from the common flying birds of the present time (e.g. terns). Most molecular dates for the divergence of Neornithes imply that they existed 40 Ma (Van Tuinen & Dyke, 2004) prior to the oldest identified ornithurine fossils in the late Cretaceous (Campanian, 80 Ma; Fountaine et al. 2005). Therefore, this would place the origins of modern birds in the early Cretaceous (120 Ma; Smith & Peterson, 2002). This later date is still earlier than that estimated from the fossil records but establishing accurate calibration times for molecular phylogenies on the basis of fossil data is a difficult task. However, the recent discovery of a close relative of ducks (Anseriformes) in the Maastrichtian of Antarctica (70 Ma; Clarke et al. 2005) indicates that Neornithes originated long before the Cretaceous/Tertiary boundary, probably earlier than 80 Ma, even if they diversified later, during the early Cenozoic (65 Ma; Zhou, 2004).
We do not know whether the fossil taxa closer to the most recent common ancestor of Neornithes than I. dispar have teeth. Therefore, we can reasonably estimate that tooth loss in crown Aves arose maximally on the stem lineage between I. dispar and Neornithes, and minimally in the most recent common ancestor of Neornithes, the origin of modern birds, i.e. approximately 100 Ma.
What the developmental genetics tells us
It has been known for more than a century and a half that transient epithelium thickenings, homologous to the dental lamina stage in the mouse, appear in the chick oral cavity at embryonic day (E)5 (Geoffroy Saint-Hilaire, 1820; Blanchard, 1860; Gardiner, 1884; Röse, 1892; Carlsson, 1896). This could be interpreted as the ‘remnants’ of an ancestral toothed condition. In birds, early tooth development arrest could be the consequence of mutations that inactivated the genetic pathways leading to tooth formation. In the last few decades, taking advantage of recent technical advances, several scientists have attempted to stimulate the ‘dormant’ odontogenic pathway in chicken with the ultimate goal of resuscitating teeth. This dream seemed attainable through elegant experiments involving either mouse/chick tissue recombinations aiming to reinitialize epithelial/mesenchymal interactions or using beads impregnated with various signalling molecules to mimic as closely as possible such interactions. The results of these experiments are summarized below and schematically analysed in Fig. 3.
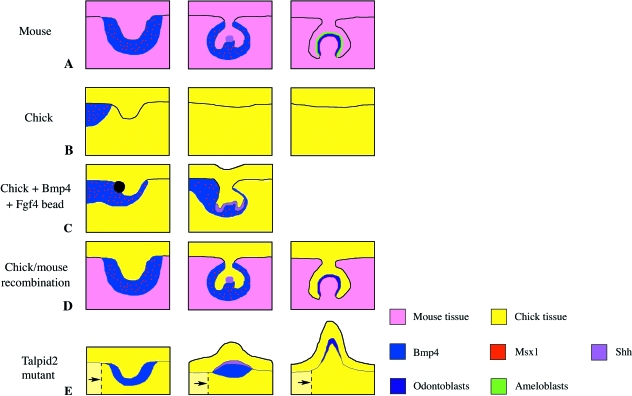
Fig. 3.
A shift in the positioning of the odontogenic epithelium relative to the dental competent mesenchyme could explain the loss of the ability to form teeth in the modern bird ancestor. Schematic drawings summarizing the chick tooth experiments. (A) Mouse molar developmental stages, from bud [embryonic day (E)12.5] to cap (E14.5) to bell (E16.5). The condensing mesenchyme around the bud stage tooth germ expresses Bmp4 and Msx1 and induces development of the enamel knot at the cap stage, which expresses signalling molecules such as Shh. The inner enamel epithelium forms the ameloblasts that form enamel, whereas the adjacent mesenchyme forms the odontoblasts that form dentine (see Caton & Tucker, 2009). (B) Chick development. At Hamburger & Hamilton (HH) stage 28 a bud-like thickening of the oral epithelium is observed. Expression of Bmp4 and Msx1 is not, however, associated with this region. No further tooth development is observed at later stages and the thickening regresses. Note that, at an earlier stage (stage 24), Bmp4 expression is epithelial and shifts into the mesenchyme at stage 28 (Francis-West et al. 1994). (C) When a bead impregnated with Bmp4 and Fgf4 is implanted into the chick epithelium, the expression of Bmp4 and Msx1 in the mesenchyme extends around the developing tooth bud. This leads to the extension and folding of the bud epithelium, and induction of Shh. No further progression of the tooth germs is observed, however (Chen et al. 2000). (D) When mouse mesenchyme is combined with chick epithelium (either by recombination of mandible tissue or by earlier neural crest grafts of mouse neural crest into a chick embryo), the chick epithelium induces Msx1 and Bmp4 in the mouse mesenchyme. The tooth germ progresses to the cap stage and forms an enamel knot-like structure expressing Shh. The mouse tissue differentiates into odontoblasts and forms a bell stage tooth germ. Tooth differentiation does not proceed beyond this stage and enamel is not deposited (Wang et al. 1998; Mitsiadis et al. 2003). (E) In the chick mutant talpid2 a shift in the positioning of the epithelium and mesenchyme has been described (indicated by dashed lines and arrows). The chick epithelium is able to induce expression of Bmp4 in the underlying mesenchyme and expresses Shh. The tooth germ develops by evagination, similar to that observed in alligator embryos. At later stages differentiated odontoblasts are identified by histology but no further differentiation occurs (Harris et al. 2006).
Kollar & Fisher (1980) performed a simple experiment in which they recombined dental epithelium of E5 chick embryos with molar mesenchyme of E16–E18 mouse embryos. These recombinant tissues were cultivated within the anterior chamber of a mouse eye. Several days later they obtained teeth with a dentine cone and an enamel cover, the famous ‘hen's teeth’. This meant that the cells of the E5 chick dental epithelium not only had retained the genetic potential to respond to the induction from the mouse mesenchymal cells for more than 100 Ma (tooth loss in a Neornithe ancestor) but they were also able to develop until the last developmental step (enamel matrix deposition). However, a possible contamination of mouse mesenchyme by mouse epithelium makes such interpretation uncertain. Indeed, it is difficult to completely eliminate contamination of mouse mesenchyme with residual epithelium and such a contamination would allow tooth formation (Arechaga et al. 1983). Eighteen years later, in another series of recombination experiments, Kollar's group showed that chick epithelium was able to induce cell proliferation and the expression of key developmental genes (Msx1, Msx2 and Bmp4) in the mouse mesenchyme, leading to odontoblast differentiation and formation of tooth germs (Wang et al. 1998) (Fig. 3D).
Another important finding was obtained by Chen et al. (2000) who showed that the early odontogenic pathway remained inducible in chick mandibles. During mandible development, they analysed the expression of crucial genes known to regulate mouse tooth morphogenesis. They discovered that the main markers for dental lamina formation in the mouse are expressed in the developing chick mandible (Fgf8, Pitx2, Pax9, Barx1, Msx1 and Msx2). However, the expression of three key genes (Bmp4, Msx1 and Msx2) was missing in the distal mandibular mesenchyme facing the presumptive chick dental lamina (Fig. 3B). When beads impregnated with Bmp4 and Fgf4 were implanted into the epithelium, facing cells in the distal mandibular mesenchyme expressed Msx1 and Msx2 (Fig. 3C). These experiments suggest not only that the odontogenic signalling pathway is conserved in the chick and can be reactivated but also that a defect in Bmp4 signalling could be responsible for the lack of Msx1 and Msx2 expression. A defect in Bmp4 signalling may therefore have occurred in a Neornithe ancestor, leading to a premature arrest of tooth development. However, exogenous addition of Bmp4, even in the presence of Fgfs, did not allow tooth development in the chick to proceed beyond the cap stage, i.e. to the stages of tooth germ differentiation. Structures that could be defined as teeth were not formed (Chen et al. 2000).
These findings obtained in vitro were confirmed in vivo by Mitsiadis et al. (2003) who performed transplantations of mouse (E8) neural crest cells in 1-day-old chick embryos. They showed that the avian dental epithelium was still able to induce a non-avian developmental programme in mouse neural crest-derived mesenchyme, resulting in tooth germ formation. Here again they did not obtain functional teeth but tooth germs developed until an advanced stage of differentiation in which some dentine-like matrix was deposited by the ectomesenchymal cells (odontoblast-like cells) and started to mineralize (Fig. 3D). No enamel-like structure was observed, however. This in-vivo study also indicates that endogenous factors coming from the mouse ectomesenchyme are more efficient at supporting tooth formation beyond the cap stage than exogenous factors used in the previous study (Chen et al. 2000). Unfortunately, the duration of these experiments was too short to determine whether or not tooth differentiation would have eventually reached a more advanced stage and the problem remains as to what extent tooth programmes are maintained in the chicken.
Recent observations made in a mutant chick [talpid2 (ta2) affected gene unknown], in which the development of several organ systems is affected, have brought additional light to the investigation on the mechanisms underlying tooth loss in birds. Indeed, the ta2 mutant was shown to develop rudimentary teeth under the rhamphotheca (Harris et al. 2006). These outgrowths from the distal mandible were conical and caniniform-shaped, morphological features that were similar to the so-called rudimentary teeth that develop in crocodile embryos. In the latter, these teeth do not develop further than the stage of dentine deposition and degenerate without any enamel covering being deposited. In contrast to the ta2 mutant, in crocodilians and lepidosaurians (lizards and snakes) when the rudimentary teeth are degenerating, first-generation teeth start to form, which are functional at hatching (and covered with enamel). Unfortunately, the oldest ta2 mutant embryos died at E16, several days prior to hatching, and so further developmental stages beyond the early dentine-like deposition were not available. It is therefore not possible to determine whether or not these rudimentary teeth would have degenerated and been replaced, as in crocodile embryos, by functional teeth. In ta2 mutants, genes necessary for tooth formation in the mouse are expressed in the mandible, including Bmp4 (Fig. 3E). The formation of advanced tooth germ in ta2 mutants is associated with defects in the specification of the oral/aboral boundary. This leads to a developmental repositioning of the presumptive dental epithelium to overlie mesenchyme competent to form teeth. Therefore, the authors proposed that changes in the relative position of a lateral signalling centre over competent odontogenic mesenchyme led to the loss of the ability to form teeth in an ancestral Neornithe.
In summary, these elegant experiments provide strong support for the mechanism of tooth loss in birds being a consequence of a developmental shift in the oral epithelium. This resulted in signalling molecules from the epithelium no longer reaching their targets in the mesenchyme and leading to a failure in the induction of key molecules, such as Msx1 and Bmp4, and an early arrest of tooth development. These experiments also indicate that, under appropriate conditions, the odontogenic capacity of the chicken dental epithelium can be reactivated. However, if the reactivation of such odontogenic pathways is a prere-quisite to initiate tooth development and to proceed further until tooth differentiation, it appears insufficient to form true functional teeth, i.e. a dentine cone covered with enamel. At the end of the developmental pathway, genes encoding for structural proteins might be activated but it appears that they were not activated in any of the experiments. In fact, they could not. Indeed, all of the genes encoding dental-specific proteins (dentine sialophosphoprotein for dentine; amelogenin, ameloblastin and enamelin for enamel) have disappeared from the chicken genome after chromosomal rearrangement or are pseudogenes (Sire et al. 2008). The revival of hen's teeth will therefore remain elusive.
Adaptations to tooth loss in birds
The fossil record tells us that the ability to form teeth was lost several times in non-avialan theropodan and avialan lineages (Sander, 1997). Three unrelated, extinct avialans lack teeth: Confuciusornis sanctus (early Cretaceous of China; approximately 120 Ma), Gobipteryx minuta and Apsaravis ukhaana (Campanian; approximately 80 Ma) (Chiappe et al. 1999, 2001; Clarke & Norell, 2002) (Fig. 2). However, the closest Avialae to modern birds is I. dispar, a toothed bird. Therefore, the loss of teeth occurred independently at least four times during Avialae evolution, including the lineage leading to modern birds. It is difficult to believe that there were four different mechanisms that led to tooth loss in Avialae and a shift in the mutual position of the competent tissues involved in epithelial/mesenchymal interactions may have been responsible in each case. However, how is it possible to survive the loss of such important tools? Indeed, we can suppose that tooth loss would have been lethal if these taxa were not already equipped with an alternative tool, the beak.
It is noteworthy that tooth loss in Avialae coincides with the presence of a beak. Indeed, although horny beaks are not preserved in fossils there are indications, for instance in the subjacent bone, of the probable presence of a rhamphotheca. Theropod and early toothed Avialae had no keratinized beak and had teeth on both the maxilla and pre-maxilla. H. regalis had a keratinized beak covering the pre-maxilla only, and no teeth in this region (Gingerich, 1975). This is a good example of a morphological intermediate structure between toothed birds, which lack a horny beak, and beaked, toothless birds. C. sanctus is the earliest bird known in the fossil record to have a toothless, horny beak, like modern birds (Fig. 2). It was hypothesized that C. sanctus fed on plant materials due to its toothless beak (Zhou & Zhang, 2003) but recent findings indicate that its diet comprised fish, like modern sea birds (Dalsätt et al. 2006).
In Avialae the horny beak represented a dramatic innovation resulting from the transformation of the reptilian snout into a beak. The rhamphotheca is assumed to have evolved from reptilian keratinized scales (e.g. Zweers et al. 1997). Interestingly, the BMP pathway was shown to be involved in the fine-tuning of beak morphoregulation in birds (Wu et al. 2004, 2006). It is speculated that this innovation was retained in Avialae because the beak compensated for the dedication of forelimbs to flight, which meant that the limbs were no longer efficient tools for finding (and manipulating) food. With beaks selected as a new tool for food uptake, the strong selective constraints on teeth were relaxed and their loss could have occurred with no drastic consequence for bird survival.
A review of non-avialan edentate tetrapodan taxa with a tentative datation of tooth loss in these lineages
A rapid glance at tetrapodan relationships (Fig. 1) indicates that tooth loss occurred independently at least seven times during evolution. Hereafter, we review the current knowledge, unfortunately often limited, of the six non-avialan edentate tetrapod taxa, i.e. toads (Lissamphibia), turtles (Sauropsida) and echidnas, anteaters, pangolins and baleen whales (Mammalia). In tetrapods, the edentate condition is not insignificant as it concerns at least 350 toads, 257 turtles, 10 000 birds and 27 mammals, i.e. one third of the currently living tetrapod species (c. 30 000 species).
Toads
Within Anura, the most successful group of living amphibians with 4500 species (Hofrichter, 2000), ‘toad’ commonly refers to a number of species of unrelated families (Bombinatoridae, Discoglossidae, Pipidae, Pelobatidae, some Microhylidae and Bufonidae) (Fig. 4). In fact, ‘true toads’ are found only in the family Bufonidae (26 genera, more than 350 species). Not a single bufonid species has teeth, whereas most members of the other ‘toad’ families possess teeth. Most frogs have an edentulous lower jaw but tiny teeth are attached to the upper jaw (pre-maxillary and maxillary teeth) and on the palate (vomerine teeth) (Cannatella & Hillis, 1993; Cannatella & Graybeal, 1996). Only Gastrotheca guentheri is known to possess teeth on the lower jaw. The teeth are homodont (i.e. all teeth similar), bicuspid or monocuspid, and divided into a distal crown and a proximal pedicel, separated by a non-mineralized fibrous ring similar to that described in the teeth of many species of the two other lissamphibian lineages, Caudata and Gymnophiona (reviewed in Davit-Béal et al. 2007).
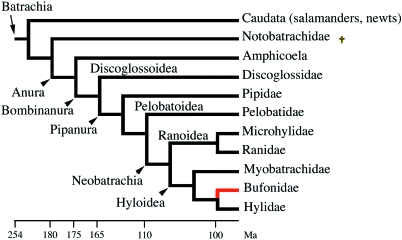
Fig. 4.
Simplified phylogeny of Batracia (after Marjanovic & Laurin, 2007). Red line: edentate toad lineage. It is worth noting that several species in various anuran lineages have also lost the ability to form teeth independently (not shown). †Extinct lineage.
What the fossil record tells us
The origins of extant amphibians (Lissamphibia) are still largely debated. The fossil record indicates that they probably arose in the Permian (about 260 Ma) (Marjanovic & Laurin, 2007; Anderson et al. 2008) but molecular data infer an older date (Carboniferous, 337 Ma; Zhang et al. 2005). The earliest known Anura and Caudata date back to the Mesozoic (early Jurassic period, 190 Ma; Shubin & Jenkins, 1995) but fossil Bufonidae are only known from Paleogene deposits (70–30 Ma) (Estes & Reig, 1973; Marjanovic & Laurin, 2007). Records of fossil anurans from the early Cretaceous indicate that the lower jaw was edentulous (Gao & Chen, 2004). Within the Hyloidea, Bufonidae are the sister group of a clade comprising Telmatobinae and Hylidae (Fig. 4), which have teeth. This means that the ability to form teeth in the oral cavity was lost in the common ancestor of modern bufonids (100–70 Ma).
Adaptations to tooth loss in toads
Toads are carnivorous, feeding principally on insects, worms, spiders and other invertebrates. The lack of teeth would seem, therefore, an important handicap for these animals but this large range of diet demonstrates that it is not. Indeed, anurans have developed an efficient tool: a protractile tongue covered with a sticky substance, making it a trap for prey. It also produces mucus to help in swallowing. This organ was already present and probably useful (as we can judge by its presence in the current sister lineage, the toothed tree frogs, Fig. 4) in the common ancestor of modern bufonids. In the few toothed anuran species, in which the oral cavity has been studied at the histological level, the oral epithelium is stratified, highly folded, thick and mucous, and shows a well-elaborated tridimensional architecture (e.g. Albright & Skobe, 1965; Loo et al. 1980). Only the very tip of the tooth pierces this epithelium, suggesting that teeth in frogs are not that efficient in aiding food uptake and processing, making them less governed by selective constraints. Their loss could have no lethal consequence (as in toads), as it would be largely compensated by the powerful tool that is their sticky tongue for food uptake. The complete lack of teeth is tentatively considered a synapomorphy of Bufonidae by most authors (e.g. Ford & Cannatella, 1993) but there are also toothless species in other anuran families (e.g. some leptodactylids; Lynch, 1970). It is worth noting that many frogs have developed odontoid elements (i.e. simple tooth-like projections of bone) on various bones and even on the lower jaw (Trueb, 1973; Shaw & Ellis, 1989). These odontoids (sometimes called fangs) could be adaptations for prey handling, which occurred as convergent evolutionary events in various lineages (Fabrezi & Emerson, 2003). The genetic processes underlying odontoid development need further investigation because their bony composition suggests that they result from different tissue interactions than those involved in tooth formation.
The edentate condition in true toads and in some other anuran taxa indicates that (i) the ability to form teeth can be easily lost and (ii) an alternative tool (the tongue), which probably arose prior to tooth loss, has been selected for during evolution. The developmental changes underlying tooth loss in toads, however, remain to be found. When considering that the ability to form teeth on the lower jaw was lost long ago in anurans, we can postulate that this condition was already that of bufonid ancestors, i.e. prior to tooth loss on the upper jaw. Therefore, the loss of ability to form teeth may have occurred in several steps, i.e. first tooth formation arrested in the lower jaw and then in the upper jaw and vomerine region. The same mechanism was probably involved but it remains to be understood. Further studies could be undertaken as for instance gene expression comparison in the upper (toothed) vs. lower (toothless) jaw of clawed toads, Xenopus laevisorX. tropicalis, or in the upper (toothed) jaw of these vs. the upper (toothless) jaw of a true toad.
Turtles
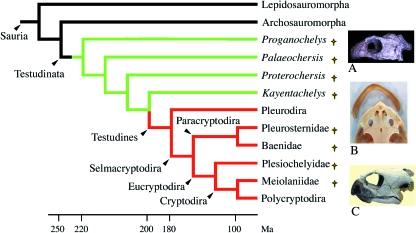
Fossil and living turtles constitute Testudinata (sensu Joyce, 2007). The living turtles are distributed within Pleurodira (side-neck turtles) and Cryptodira (hidden neck turtles) with a total of 257 living species and 12 families (Ernst & Barbour, 1992). The monophyly of Testudinata has never been questioned and all living species are toothless (Fig. 5).
Fig. 5.
Simplified turtle relationships (after Gaffney & Meylan, 1988; Rieppel, 1999; Joyce, 2007). Green lines: lineages with palatine teeth only; red lines: toothless lineages. (A) Skull of Proganochelys quenstedti (from Gaffney, 1990). (B) Dorsal view of the skull and beak of the snapping turtle (aquatic). (C) Skull and beak of a terrestrial turtle. †Extinct lineages.
What the fossil record tells us
The origin of turtles from ancestral sauropsids is still unclear and largely debated. Molecular data are partially congruent with morphological characters supporting diapsid rather than anapsid turtle relationships [Rieppel & deBraga, 1996; deBraga & Rieppel, 1997; see Laurin & Reisz, 1995 and Lee, 1997 for Parareptilia (anapsid) turtle relationships]. However, the molecular data conflict with palaeontological data as to where exactly turtles fit within diapsids (Rieppel, 1999). Phylogenetic studies either place turtles close to the lepidosaumorphs (tuatara, snakes and lizards) (e.g. Hill, 2005) or close to the archosauromorphs (crocodiles and birds) (e.g. Hedges & Poling, 1999; Iwabe et al. 2005). The turtle ancestor diverged from the other diapsids between 285 and 270 Ma (McGeoch & Gatherer, 2005) but its origin remains a mystery.
The most ancient and well-known turtle is Proganochelys quenstedti(late Triassic, 220 Ma). It shows primitive features absent from modern turtles that make it useful for understanding turtle evolution (Gaffney, 1990).P. quenstedti was roughly similar to the species that live today, except for, among other characters, the presence of several rows of conical teeth on the vomers, palatines and pterygoids (Fig. 6D), which make it unique among Testudinata as the other ancient turtles lack these teeth (Joyce, 2007). The maxilla, pre-maxilla and dentary are edentulous but the pre-maxillary has tooth vestiges (Kordikova, 2002). Among amniotes the presence of vomerine and palatine teeth is widespread. This is interpreted as the primitive condition in Testudinata. Another fossil turtle, Proterochersis robusta (late Triassic) was probably contemporary to P. quenstedti. P. robusta possessed several rows of small pterygoid teeth and shared several features of pleurodires such as the pelvis fused into the shell. Its affinities to pleurodires, however, are not certain. Palaeochersis talampayensis (late Triassic of Argentina) appears closer to all other turtles than P. quenstedti and P. robusta but still outside the common ancestor of cryptodires and pleurodires (Rougier et al. 1995; Joyce et al. 2004; Joyce, 2007).
Fig. 6.
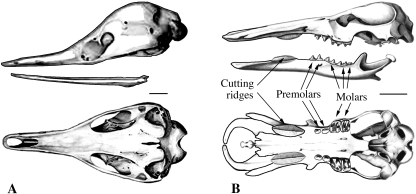
(A and C) Lateral and ventral views of the skull of a primitive tetrapod, the parareptilian Procolophon. (B and D) Lateral and ventral views of the skull of Proganochelys quenstedti. (E) Ventral view of the skull of Kayentachelys aprix. Small dots represent teeth. M, maxillary; Pal, palatine; Pm, pre-maxillary; Pt, pterygoid; V, vomer. (A and C) From Carroll & Lindsay (1985). (B and D) From Gaffney (1990). (E) From Gaffney et al. (1987). Scale bars, 1 cm.
Unquestionable pleurodire skulls are not observed until the early Cretaceous (145 Ma). From this period onwards, Pleurodira lack palatine teeth. The oldest turtle, recognized by some authors as a Cryptodira, is Kayentachelys aprix (late Jurassic, 150 Ma), which possessed pterygoid teeth (Fig. 6E). The position of K. aprix, however, is still questioned and it is put outside the common ancestor of cryptodires and pleurodires by Joyce (2007). All other Cryptodira lack teeth. The pleurodire/cryptodire dichotomy probably took place during the early Jurassic (200–180 Ma). Depending on the position of K. aprix in the tree, palate teeth could have been lost either independently in Pleurodira and Cryptodira (Gaffney, 1990) or in the common ancestor of pleurodires and cryptodires. In ancient turtles, palatine teeth are characterized by a primitive, thecodont implantation, in which teeth are set in sockets that are distinct from the surrounding bone (Kordikova, 2002).
Adaptations to tooth loss in turtles
All living turtles, aquatic and terrestrial, have a keratinized beak (Fig. 6B,C). Although the common ancestor of all living turtles was aquatic, the earliest turtles clearly lived in a terrestrial environment (Joyce & Gauthier, 2004; Scheyer & Sander, 2007). The origin of the beak in turtles was traced back as early as P. quenstedti, which had toothless jaws but already possessed a rigid beak (the presence of a keratinized beak is suggested by the condition of the mandibles). Turtle beaks have sharp edges for cutting food and most have strong jaws, which they use to tear food and capture prey.
Hatchlings emerge from their eggs using what is commonly known as the egg tooth or caruncle. It is located at the front of the upper jaw and disappears a few months after hatching. This is a modified scale (i.e. a keratinized structure derived from epithelial cells) and not a real tooth.
The beak covers the upper and lower jaws and is ornamented by horny ridges, similar to the beak of birds. Various adaptations of the beak and oral cavity have occurred by means of natural selection (knife-sharp ridges for carnivorous species, ridges with serrated edges for plant-eating species, bony ridges helping to crush mollusc shells) that permitted turtles to utilize a large food spectrum (leaves, fruit, mushrooms, insects, snails, crayfish, fish and jelly fish). Turtles use their tongues in swallowing food.
It seems clear from the fossil record that the ability to form teeth on the upper and lower jaw was lost before the origin of Testudinata, i.e. approximately 250–220 Ma. At this epoch, the teeth were widespread in the oral cavity of early reptiles. We postulate that early Testudinata (yet to be found in the fossil records) possessed teeth fixed on the upper and lower jaw bones and on various bones of the palate. Teeth located on the pre-maxillary, maxillary and dentary were subsequently lost during Testudinata evolution but before the first Testudinata found in the fossil record, approximately 220 Ma. As suggested for birds, the presence of a keratinized beak that was efficient for food uptake probably relaxed the functional pressure on teeth, which were probably lost through a similar process to that described in birds (see above). In the turtle's ancestor, as in the bird's ancestor, the beak minimized the negative consequences of tooth loss.
In turtles, teeth were retained on the palate longer than in jaws. Teeth were lost in the vomers and palatines first, then later on the pterygoids. It appears that this loss was not a dramatic event as this has occurred independently in several vertebrate lineages during evolution. For instance, the Archosauria (living crocodiles and birds but also extinct non-avian dinosaurs, pterosaurs and relatives of crocodiles) have no teeth on the pterygoid, palatine or vomer.
Echidnas
Monotremes form a single order, Monotremata. These egg-laying mammals are the survivors of an early branching of the mammal tree that diverged from the other mammalian lineage, Theria, about 225 Ma (molecular data; Westerman & Edwards, 1992; van Rheede et al. 2006) (Fig. 1). Monotremata have retained reptilian characters, such as oviparity and a therapsid-like shoulder girdle, that have been lost in therian lineages. Extant and extinct monotremes are composed of four families, two extinct (Kollikodontidae and Steropodontidae, both from the early Cretaceous, 110 Ma) and two still represented in nature, the semi-aquatic Ornithorhynchidae (platypus) and the terrestrial Tachyglossidae (echidnas) (Musser & Archer, 1998). To date, monotremes are all indigenous to Australia and New Guinea but they were more spread in the past as revealed by the presence of a fossil platypus (Obdurodon) in the middle Miocene (10 Ma) of Argentina (Pascual et al. 1992; Musser & Archer, 1998).
Extant tachyglossids include the short-beaked echidna, Tachyglossus aculeatus, living in Australia and New Guinea, and three species of the genus Zaglossus, the long-beaked echidnas Z. brujinii, Z. bartoni and Z. attenboroughi, in New Guinea. The most prominent feature of the head is the elongate, hairless and rounded snout (Fig. 7A). This tubular, elongated snout has a small and narrow opening. Juvenile and adult echidnas are edentulous and the lower jaw is extremely reduced. However, the hatchling possesses an egg tooth on the snout (a true tooth as in lizards and snakes) that is a useful tool to pierce the keratinized egg shell. This is the only tooth the echidna will ever possess and it is lost after hatching. However, it is worth noting that this egg tooth is crucial for echidna survival. This explains why, although echidnas have been toothless for many millions of years, there are still strong functional constraints acting on dental proteins (Sire, unpublished data).
Fig. 7.
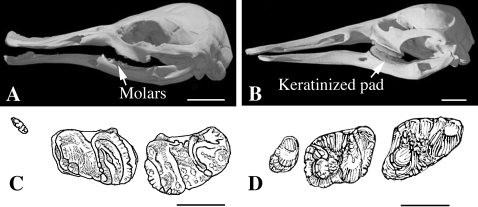
Lateral and ventral views of the skull of Monotremata. (A) The echidna, Tachyglossus aculeatus. (B) A fossil (adult) ornithorhynchid, Obdurodon dicksoni (after Musser & Archer, 1998). In this species the skull morphology is very similar to that in platypus (see Fig. 13), except for the presence of teeth in adults (two pre-molars and two or three molars). Scale bars: A, 1 cm; B, 2 cm.
What the fossil record tells us
Ornithorhynchidae are known from the Cretaceous (120 Ma), whereas Tachyglossidae seem younger, as they are only found in the mid-Tertiary (25 Ma). Their relationships are not clear. There are several arguments that suggest that echidnas are derived from a platypus-like ancestor but they may have evolved from an unknown, more generalized Monotremata (Musser, 2003). The divergence between the two lineages is estimated to have occurred by the end of the Cretaceous (80–70 Ma; Rowe et al. 2008).
Fossil tachyglossids, such as Megalibgwilia robustafrom the middle Miocene (15 Ma; Griffiths et al. 1991), are similar in appearance to the living Z. bruijnii, although larger, and were probably able to consume larger prey. The skull had a long, sturdy snout and the palate possessed a groove to accommodate tongue extension. There were no teeth. The largest extinct echidna Z. hacketti is known only from a few bones found in Western Australia (Pleistocene, 0.5 Ma).
Given that ornithorhynchids have teeth (see below), it appears that the ability to form jaw teeth in tachyglossids was lost after the echidna ancestor diverged from the platypus ancestor, i.e. by the end of the Cretaceous/beginning of the Tertiary (70 Ma).
Adaptations to tooth loss in echidnas
Echidnas are insectivores. The diet of T. aculeatusconsists of ants and termites, whereas Z. bruijnii prefers insect larvae. They use their long sticky tongue to catch prey from its hole. Capturing and grinding prey with the tongue occur due to a unique jaw mechanism; the echidnas open and close their mouth by rotating their mandibles around their long axes (Murray, 1981). Histological observations and scanning electron microscopy of the palatal epithelium of T. aculeatus revealed the presence of keratinized palatal spines. It is suggested that these horny teeth-like projections are highly differentiated filiform papillae, which have developed as a compensatory mechanism of mastication, as this animal is edentulous (Doran, 1975).
The large temporal gap between the earliest echidna in the fossil record (around 25 Ma) and the molecular datation of the differentiation of the lineage (estimated at 70 Ma) does not allow for clear relationships to be defined. However, we can speculate about the events that might have led to tooth loss. It appears that the earliest toothed monotremes, such as kallikodontids from the early Cretaceous (110 Ma; Flannery et al. 1995), were beaked species. Again, as described for birds and turtles (see above), it seems that the presence of a keratinized beak prior to tooth loss could account for relaxing the functional constraints on teeth. Simultaneously, the elongated morphogenesis of the lower jaw could have reduced the ability of the jaw to accommodate teeth. Snout elongation (like in anteaters, see below) was probably concomitant with the presence of an elongate tongue, a tool that compensated, along with the beak, for tooth loss. Further adaptations improving food processing, such as the spines on the tongue, developed later through natural selection.
Anteaters
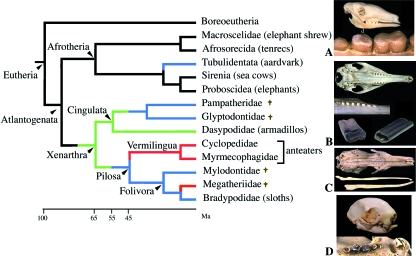
Anteaters belong to Xenarthra, which are principally characterized from the other placentals in having additional vertebral articulations (one of the five skeletal synapomorphies of Xenarthra). The clade Xenarthra is composed of Cingulata (shelled Xenarthra, armadillos and extinct pampatheres and glyptodonts) and Pilosa [composed of Vermilingua, anteaters and Folivora (arboreal sloths and their extinct relatives)] (Fig. 8). The 31 species of living xenarthrans are distributed into five families, all of which are restricted to Central and South America (with the exception of one species, the nine-banded armadillo, which ranges north to the USA). The five families include the Dasypodidae (armadillos, 21 species), Myrmecophagidae and Cyclopedidae (anteaters, four species), Bradypodidae (three-toed sloths, four species) and Megalonychidae (two-toed sloths, two species) (Vizcaíno, 1995; Anderson & Handley, 2001). Anteaters are toothless, whereas sloths lack enamel on their teeth and, in armadillos, enamel is present only in the first generation of teeth, at least in the nine-banded armadillo (see below). In order to better understand the origin of the dental features observed in Xenarthra, prior to dealing with anteaters specifically, we briefly review our current knowledge on Xenarthra origin and on the evolution of their dentition.
Fig. 8.
Simplified phylogeny of Xenarthra and Afrotheria (after Hallström et al. 2007; Seiffert, 2007). Red lines: toothless lineages; blue lines: enamel-less lineages; green lines: enamel reduction. (A) Lateral view of the skull and detail of the upper cheek teeth of an aardvark, Orycteropus afer. (B) Ventral view of the skull and of the upper right jaw, and detail of extracted teeth of a nine-banded armadillo, Dasypus novemdelineatus. (C) Ventral view of the skull of a giant anteater, Myrmecophaga tridactyla. (D) Ventral view of the skull and detail of the upper jaw of a three-toed sloth. †Extinct lineages.
What the fossil record tells us
Since the redistribution of the species, which comprised the polyphyletic Edentata into the three, now undisputed, monophyletic clades, Xenarthra, Tubulidentata and Pholidota, xenarthrans are considered as a major clade of placental mammals (e.g. Murphy et al. 2001; Madsen et al. 2001; Delsuc et al. 2002, 2004; Möller-Krull et al. 2007). However, their position among placentals is still in debate, as illustrated by two recent studies based on large data sets of nuclear genes (Hallström et al. 2007; Nikolaev et al. 2007). The current trends supported by these molecular phylogenies are that Xenarthra are the sister group of Afrotheria (Figs 1, 8). The probable reason for the previous uncertainty is that the divergence of the placental lineages took place a long time ago within a narrow temporal window. Molecular analyses provide estimates of the origin of Xenarthra within the late Cretaceous (100 Ma; Delsuc et al. 2004). These molecular data are, as usual, at odds with the palaeontological data, which indicate that the earliest records of Xenarthra come from the Paleocene (58–55 Ma; Scillato-Yané, 1976). However, because these fossil species show features that are already proper to this order, this strongly suggests that the origin of the xenarthrans is more ancient and probably located in the late Cretaceous/early Paleocene (80–61 Ma), a date that substantially reduces the gap between molecular and palaeontological data. It is probable that the evolutionary radiation of Xenarthra occurred around 65 Ma, after South America became isolated from the other continents (Patterson & Pascual, 1972).
In the early Paleocene (65–61 Ma) there are no fossil species that could be considered with certainty as a stem Xenarthra (e.g. proto-Xenarthra). Given their similar anatomy to extant armadillos, the Palaeanodonta are often proposed as related to the Xenarthra (Hoffstetter, 1982; Rose et al. 2004). However, palaeanodonts are also considered to be closer to Pholidota (pangolins) than to the other lineages (see below). It is believed that the ancestral lineages that lead to armadillos, sloths and anteaters diversified during the Eocene (55–33 Ma). However, phylogenetic relationships of modern species to fossil records are difficult and the absence of representatives of the main xenarthran lineages in the South American fossil record of the late Cretaceous/early Paleocene period is still an enigma (Carlini & Scillato-Yané, 2004).
The first group consisted of land-dwelling armoured plant-eaters, whose descendants developed into modern-day armadillos. Armadillos are the oldest true Xenarthra known in the fossil record (late Paleocene, 59–57.5 Ma; Scillato-Yané, 1976; Bergqvist et al. 2004). A second group consisted of insectivores, which specialized in ants and termites (anteaters). There are only a few fossils available for early anteaters. The oldest fossil of this group is from the early Miocene, and probably closely related to Tamandua(Carlini et al. 1992). Specimens, such as Protamandua, begin to be more abundant during the early/middle Miocene (17.5–16.3 Ma; Hirschfeld, 1976; Gaudin & Branham, 1998). Given the close relationships between Vermilingua and Folivora (sloths), anteater ancestors were certainly older and could have differentiated during the early Eocene (Pujos & De Iuliis, 2007; Pujos et al. 2007). The third group is represented by modern-day sloths, a tree-dwelling group of animals lacking armour. The oldest sloths are reported from the Eocene of Antarctica (Vizcaíno & Scillato-Yané, 1995) but most records are from the Miocene period. Recently, the description of two Pseudoglyptodon species from the early and late Oligocene (36–24.5 Ma) revealed that this typical ground sloth shared several dental characters with armoured Glyptodontoidea and may represent not only a pre-folivoran stage but also the earliest known Pilosa (McKenna et al. 2006; Pujos et al. 2007; Pujos & De Iuliis, 2007).
Evolution of the dentition in Xenarthra
The condition of xenarthran ancestors was a continuous dentition, including incisors and canines (Hoffstetter, 1982). However, in all true Xenarthra fossils known to date, the upper teeth are only located on the maxilla, the pre-maxilla being always edentulous. The early fossils (early Miocene; Carlini et al. 1992) relating to Vermilingua (anteaters) lack teeth (a synapomorphy of this group) and a study of the skull structure in Neotamandua and Palaeomyrmidon clearly shows that the fundamental specializations of the skull, related to ant and termite eating, had been achieved prior to the Miocene.
The general dental morphology of extant and extinct Cingulata (armadillos) and Folivora (sloths) is different from that of the other placental mammals, including their closest relatives, the Afrotheria, i.e. absence of the most anterior teeth, incisors and canines; presence of a few molariform cheek teeth (‘cheek’ means that pre-molars and molars are hardly morphologically distinguishable one from another), which are cylindrical, generally homodont in Cingulata but heterodont (i.e. teeth of various shapes such as incisors, canines and molars) in Folivora, and hypsodont (i.e. teeth with a high crown and tall with respect to their occlusal area) and hypselodont (ever growing with open roots). In addition to lacking enamel (see the armadillo description), xenarthran teeth are built of different dentine tissues (Vizcaíno & Scillato-Yané, 1995). In a comparative study of xenarthran dental structure, Ferigolo (1985) described the teeth as being generally composed of a small layer of cementum covering the dentine crown that is constituted of a hard layer of modified orthodentine (osteodentine in some Glyptodontidae). The upper region of the pulp cavity is occupied by vasodentine. There is a large difference in resistance to wear between the hard outer and soft inner layers of dentine. Vasodentine is absent from non-abrased teeth, which means that either this typical dentine is deposited late, after tooth eruption, or is a reaction to abrasion and contributes to the protection of the pulp cavity from external pathogens.
There is no information on teeth in the oldest Cingulata from the late Paleocene but subsequent representatives already show the dental characters of living species and a similar statement is true for the early fossil sloths recorded. This means that ever-growing, high-crowned teeth differentiated early in the evolution of these lineages, i.e. probably during late Cretaceous/early Paleocene, and that the adaptation to plant feeding was secondary to this condition.
The Xenarthra appear marked by strong disadvantages, with a reduced dentition and thin enamel that would have provided poor tooth protection against abrasion. Therefore, one would have expected rapid extinction, rather than the observed rapid radiation. Apparently, early Xenarthra found, in the recently geographically isolated South America, free niches, which allowed their diversification, with hypsodonty and hypselodonty permitting teeth to compensate for tooth erosion.
Anteaters have lost the ability to form functional teeth
The four living species of anteater (giant and pygmy anteaters, and two tamanduas) and all of their fossil relatives are toothless. The ability to form functional teeth was lost long before the Miocene period (i.e. > 25 Ma) but in tropical forests fossilization is not favourable and skeletal remains of Vermilingua from earlier periods have probably not been conserved.
Although anteaters have no functional teeth, tooth germs have been described in anteater embryos, in many if not all situations. These remnants of teeth, however, are resorbed prior to birth (Peyer 1968). This is evidence that Vermilingua ancestors had functional teeth and that early odontogenetic processes are conserved long after the ability to form functional teeth is lost, as observed in modern birds (see above).
Adaptations to tooth loss in anteaters
Anteaters ingest food without chewing using a slender, elongated tongue, which can project to a distance greater than the cranial length. The tongue is ornamented with little keratinized spines that point backwards and is covered with sticky saliva. A large and elongated hyoid apparatus, with articular surfaces permitting great freedom of movement, supports the tongue. The hyoid muscle enables the tongue to project out with great speed and precise positional control. This feature combines with an elongated secondary palate, accommodating the retracted tongue within the oropharynx. The giant anteater, Myrmecophaga tridactyla, has a long and thin head, and its tongue is 60 cm in length and can extend and retract at the incredible rate of 150 times per minute. With their special insect-catching tongue, anteaters can eat thousands of ants or termites in a day. Instead of teeth, edentulous anteaters have horny protrusions called papillae on the roofs of their mouths and strong, muscular stomachs (Vizcaíno & Loughry, 2008).
When considering the features of the dentition in Xenarthra (but see later and Fig. 8), it appears that (i) these highly adapted features in anteaters (especially the skull and tongue) were acquired after the Vermilingua differentiated from the Tardigrada, and (ii) the ancestral condition in the Vermilingua was a reduced dentition with hypsodont and hypselodont teeth either covered or not with enamel and a diet based on insects. One can also imagine that a sticky tongue was present before the ability to form teeth was lost (as also supposed for the Pholidota ancestor, see below). We can deduce that the absence of an efficient protective layer of enamel (no enamel or only a thin layer that is rapidly worn away) led to strong abrasion of the teeth. This excessive abrasion could have resulted in a specialization towards a diet that does not necessitate teeth (ants and termites) and the presence of a sticky tongue was therefore necessary to fulfil this function. Given such a specific adaptation, the functional constraint on teeth was relaxed, allowing for the loss of teeth, perhaps through changes in the odontogenic pathways resulting, for instance, from the shift of competent odontogenic territories linked to a change in the shape of the jaw.
Pangolins
Pangolins, also called scaly anteaters, have their back covered with large overlapping keratinized scales made up of agglutinated hairs. They constitute the clade Pholidota, with a single family (Manidae) and a single genus (Manis) including several extinct species. Eight species are still living, four in Africa and four in Asia (Gaubert & Antunes, 2005).
Living pangolins and extinct manids lack teeth as adults. They have incomplete zygomatic arches and possess an extremely reduced, bladelike mandible (Fig. 9B). Each dentary has a single bony protrusion (Nowak, 1999). The ability to form functional teeth appears to have been lost in an ancestral manid. It is worth noting that vestigial teeth start to form in embryos but are resorbed prior to birth. In M. javanica, Röse (1892) described a dental lamina in the anterior part of the lower jaw and tooth germs forming rounded buds. The observation of vestigial teeth was confirmed by Tims (1908).
Fig. 9.
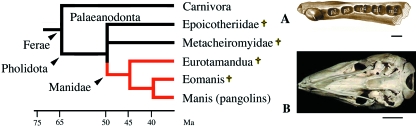
Simplified phylogeny of Pholidota (after McKenna & Bell, 1997; Nowak, 1999). Red lines: toothless lineages. (A) Dorsal view of the right dentary of a primitive palaeanodont (Eocene) showing the large canine (no incisors) and the alveoli for three pre-molars p2–p4 (p1 absent) and two molars. Modified after Rose et al. (2004). (B) Ventral view of the skull and lower jaw of a living pangolin, Manis javanica. Note the extremely narrow and weak blade-like mandible. †Extinct lineages. Scale bars: A, 1 mm; B, 1 cm.
What the fossil record tells us
The relationships of Pholidota are still debated. Phylogenetic analyses based on morphological data placed pangolins as the sister group to xenarthrans (Novacek & Wyss, 1986; Novacek, 1992a,b) and, even within them, close to anteaters. However, it seems that these close relationships are due to high anatomical convergences in relation to a similar diet. Molecular analyses based on large data sets clearly indicate that Pholidota is the sister group to Carnivora (Murphy et al. 2001; Scally et al. 2001) (Fig. 9).
Modern-looking fossil pangolins (e.g. Eomanisand Eurotamandua) were found in the middle Eocene of Germany (40 Ma; Horovitz et al. 2005). A single fossil was found in the lower Oligocene of North America (30 Ma) and more recent remains of scaly anteaters come from the Pliocene of Europe, the Plio-Pleistocene of South Africa and the Pleistocene of Asia (Emry, 1970).
Palaeontological studies support that manids are possibly related to Palaeanodonta, a group of extinct toothed anteater-like mammals that lived from the late Paleocene (55 Ma) to the early Oligocene (35 Ma; Gunnel & Gingerich, 1993) (Fig. 9). Both manids and palaeanodonts constitute the Pholidota. The best-known extinct Pholidota is Metacheiromys, from the mid-Eocene of North America, that already looked like a modern species (Simpson et al. 1931). Palaeanodonts had a reduced dentition with no incisors, one large canine and five to eight post-canine teeth not strongly rooted in the jaw (Fig. 9A). They probably fed mainly on ants and termites as suggested by the morphology of their post-canines. In some palaeanodonts the post-canines are covered with enamel (e.g. epoicotheriids), whereas others have no enamel or only a thin layer that is rapidly worn away (e.g. metacheiromyids; Gunnel & Gingerich, 1993).
Adaptations to tooth loss in pangolins
Pangolins are covered by tough scales that protect them from their aggressive prey, ants and termites. The pangolins open the anthills and termite mounds using their long claws on their forelimbs and catch the prey with their long tongue; the myrmecophagous diet and the elongate, sticky tongue are efficient adaptations to toothlessness. Their tongue is vermiform and can reach 25–40 cm. It is covered with viscous saliva, secreted by a large salivary gland. The tongue extends to the abdominal cavity and is attached to the pelvis. In addition, the stomach possesses a muscular, gizzard-like pyloric region with keratinized spines (the so-called ‘pyloric teeth’; Krause & Lesson, 1974) and contains small stones and sand. All of the processes of grinding are done in this gizzard, compensating for the lack of teeth. The stomach lumen is lined with a cornified, stratified squamous epithelium and there are numerous glands (a mucous gland spread in three regions, an oxyntic gland and a pyloric gland) responsible for the digestion of their highly chitinous diet (Nisa et al. 2005).
It remains uncertain which developmental failure was responsible for tooth loss in the toothed ancestral Pholidota but, given the palaeontological data, this event probably occurred during the late Paleocene/early Eocene period (55–50 Ma), when the first manid ancestors differentiated. Indeed, edentate fossil manids are found as early as 40 Ma in the fossil record (Horovitz et al. 2005). We postulate that tooth loss could have been preceded by a drastic reduction of the enamel cover, as observed in some palaeanodont taxa. Such a loss of protective hard cover resulted in a strong abrasion of the teeth, as described in some species (Secord et al. 2002), and hence in a specialization towards a different diet based on insects. Whether these early manids already had a protactile sticky tongue as observed in recent species remains uncertain but such a tool could have contributed greatly to improve food uptake and compensated when the ability to develop teeth was lost.
Baleen whales
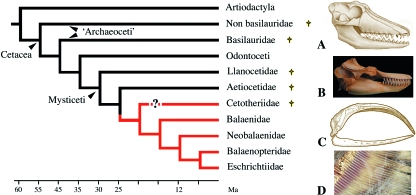
The Cetacea is composed of ‘Archaeoceti’, which is a stem polyphyletic and exclusively extinct assemblage, Odontoceti (toothed whales) and Mysticeti (baleen whales). Living odontocetes contain six families (69 species): Delphinidae, Monodontidae, Phocoenidae, Physeteridae, Platanistidae and Ziphiidae. All fossil and modern toothed whales are thought to be monophyodont, i.e. they have a single tooth generation. Most species are homodont but some extinct Odontoceti, e.g. Agorophiidae and Squalodontidae, were heterodont (Fordyce, 1982). In contrast to delphinids and phocoenids, in which teeth are numerous on both jaws, modern physeterids, ziphiids and monodontids have reduced dentitions: no teeth or unerupted teeth on the upper jaw and a few teeth on the lower jaw. Some females can even be edentate. A toothless ziphiid-like fossil has been described (Fordyce et al. 2002). Male (rarely female) narwhals (monodontids) have a single long spiralled tusk derived from the incisor on the left maxilla and a small tooth on the right maxilla; they have no teeth on the lower jaw.
Living mysticetes comprise at least 14 species distributed within four families: Balaenidae, Balaenopteridae, Neobalaenidae and Eschrichtiidae (Fig. 10). All living species of baleen whales are toothless but they derive from toothed mysticete ancestors.
Fig. 10.
Simplified phylogeny of Cetacea (after Uhen, 2002; Deméré et al. 2008). (A) Skull of an early archaeocete, Pakicetus from the early Eocene. (B) The toothed jaws of an odontocete, the orca Orcinus orca. (C) The edentulous jaw of a mysticete, the bowhead whale Balaena mysticetus. (D) Detail of a baleen plate of a gray whale Eschrichtius robustus. †Extinct lineages.
What the fossil record tells us
The origin and phylogeny of the Cetacea is still debated but most authors now agree (with a large input from molecular phylogenies) that cetaceans are nested with artiodactyls (hence the name of the new order, Cetartiodactyla) and closely related to anthracotheres, the ancestors of hippopotamuses (Gatesy et al. 1996; Nikaido et al. 1999). Modern cetaceans are supposed to have arisen from ‘Archaeoceti’, a group of early cetaceans, which were large toothed predators that appeared in the Eocene (50 Ma). The archaeocetes themselves are believed to derive from aquatic even-toed ungulates (artiodactyls) that diverged at the Cretaceous/Tertiary boundary and during the early Paleocene (60 Ma; Bajpai & Gingerich, 1998; Thewissen et al. 2007; Uhen, 2007). Archaeoceti, such as Basilosaurus from the middle Eocene, were diphyodont, i.e. they had milk teeth, shed and replaced by permanent teeth. They were also heterodont separated from the cheek teeth (pre-molars and molars) by a diastema (Uhen, 2002).
Early fossil mysticetes were also found as early as the Eocene period. They possessed teeth, although their cranial architecture was similar to that of modern baleen whales (Barnes & Sanders, 1996). Aetiocetus cotylalveus (Aetiocetidae) appears to be the most primitive mysticete and possessed a heterodont dentition on both jaws (incisors, canines, pre-molars and molars). It is considered a transitional, toothed mysticete that gave rise to toothless primitive types (Barnes, 1984). The small multicusped teeth of aetiocetids were used to filter food from the seawater rather than for selecting individual prey (Fordyce & Barnes, 1994). Aetiocetids are thought to have had rudimentary baleen plates on the upper jaw (Deméré, 2005; Deméré et al. 2008). Being made of keratin, baleens do not fossilize. However, the bones that support the baleens (i.e. the palate bones) show additional features that bear witness to the presence of baleens in toothed mysticeti from the early Miocene (20 Ma; Kellogg, 1965; Fordyce & Muizon, 2001) and even earlier as recently reported in Oligocene aetiocetids by Deméré et al. (2008). These authors described the simultaneous presence of teeth and baleens on the jaw, which supports a progressive transition from a condition with teeth to a condition with baleens during further evolution in mysticetes. Such a transition is still observed in the embryonic series of modern species. Indeed, although mysticetes are toothless at birth, tooth buds form and grow in the embryos, then baleens develop and the teeth are resorbed (Karlsen, 1962).
Most post-Oligocene mysticetes are baleen-wearing and toothless (Fordyce & Barnes, 1994). The most ancient family of edentulous mysticetes are the Cetotheriidae that lived from late Oligocene (25 Ma) to Pliocene (5 Ma). They have been positioned close to Balaenopteridae but their relationships with modern Mysticeti are unclear (Uhen, 2002) (Fig. 10).
Vestigial teeth in baleen whale embryos
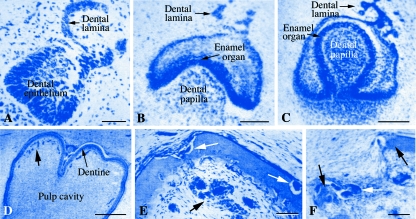
The presence of tooth buds in baleen whale embryos was first reported by Geoffroy Saint-Hilaire (1807) and Cuvier (1836) but no information was available about the structure of the hard tissues. Van Dissel-Scherft & Vervoort (1954) and then Karlsen (1962) described the successive steps of vestigial tooth development in fin whale Balaenoptera physalusembryos. A total of 110 tooth germs (53 at the upper jaw and 57 at the lower jaw) develop up to the advanced bell stage but the teeth do not erupt. It is noteworthy that 23 and 15 of these tooth germs, respectively, are double teeth developing by fusion of tooth germs. On the lower jaw, the first tooth germs develop close to Meckel's cartilage before the bone starts to ossify. In B. physalus, tooth germs start to develop in 2-cm embryos from a dental lamina and then tooth morphogenesis and differentiation take place until an advanced bell stage. The enamel organ is well differentiated and faces well-differentiated odontoblasts, which deposit pre-dentine matrix. The pre-dentine, however, does not mineralize (Fig. 11A–D). Two types of dentine are deposited by the odontoblasts: one is acellular orthodentine, whereas the other contains enclosed mesenchymal cells. Some tooth germs produce cellular dentine only, whereas both dentine types occur in others. Dentinal tubules are not present and no enamel matrix is produced. This is the most advanced stage that tooth germs can reach (in 63-cm embryos) and then they start to be resorbed. The odontoblasts disappear and the enamel organ reduces. In an 82-cm embryo, dentine resorption has started (Fig. 11E,F). In a 212-cm embryo, all tooth germs on the upper jaw have been resorbed. A marked proliferation of epithelial cells, indicating the formation of the first baleen plates (also called whalebones), is identified in the region of the tooth germs as the latter start to be resorbed. In a 330-cm embryo, tooth germs are no longer seen in the lower jaw. It is worth noting that a newborn fin whale is 6 m long.
Fig. 11.
Histological evidence of embryonic teeth developing in the embryos of the baleen whale Balaenoptera physalus. Most tooth germs attain an advanced bell stage, until dentine deposition, and are then progressively resorbed. (A–D) Tooth morphogenesis and differentiation. (E and F) Resorption. (A) Cap stage. (B) Bell stage; dental epithelium starts to fold around dental papilla cells. (C) Advanced bell stage, in which the dental epithelium entirely surrounds the dental papilla. (D) A thin layer of dentine has been deposited; enamel is not identified. The arrows point to numerous capillary blood vessels located close to the dentine layer. (E) Initiation of resorption process. White arrows indicate osteoclasts. Black arrow points to blood vessels. (F) Advanced stage of resorption. Black arrows indicate the dentine; white arrow points to osteoclasts. Modified after Karlsen (1962). Bars: A, 50 µm; B, C, E and F, 100 µm; D, 500 µm.
Working on Balaenoptera acutorostrata (a baleen whale that is 3 m long at birth), Ishikawa & Amasaki (1995) similarly found that vestigial teeth develop until the embryos reach 90 cm, then degenerate and were no longer detected in 182-cm embryos. As described above, degenerate tooth buds and rudiments of baleen plates were simultaneously present on the upper jaw, a situation that is reminiscent of the ancestral condition in Oligocene mysticetes.
Adaptations to tooth loss in baleen whales
Baleen plates are a unique filtering structure used to consume prey. The baleen plates are keratinized modified elements of the oral epithelium, rooted in and suspended from the palate and extending into the oral cavity (Utrecht, 1965). They consist of many fibrous tubules packed together to form plates (Fig. 10D), arranged in a row on either side of the mouth. The ends of the plates become frayed and interwoven, resembling a comb, creating an efficient sieve for trapping small fish and zooplankton.
Trends in tooth evolution in baleen whales can be summarized as follows. The primitive heterodont and diphyodont dentition of the ancestral aquatic artiodactyls was replaced by a homodont and monophyodont dentition (as currently observed in toothed whales). In the ancestors of mysticetes the teeth became smaller and the animals adapted to filter-feeding, which allows access to small prey. This change in feeding was facilitated by the simultaneous presence of rudimentary baleen plates. As these tools provided more efficient filtering they were positively selected during further mysticete evolution and the functional constraints were relaxed on teeth. The small teeth were no longer useful and their loss was largely compensated by the presence of baleens. This event could have occurred during the Oligocene period, around 30 Ma. It is still difficult to establish solid relationships between the Oligocene toothed baleen whales and the modern mysticete. However, it seems more parsimonious to postulate that the common ancestor (probably toothed) of all the modern mysticete lineages possessed baleens rather than baleens appearing independently in these lineages.
In modern mysticetes there is a close relationship between the vestigial tooth bud being resorbed and baleen plate development, and we could speculate that the development of the baleen plates could have played a role in tooth loss. However, baleen plates and functional teeth were simultaneously present, in the same locus, in the upper jaw of archaic mysticetes. The process that could have led to the loss of the ability to form teeth in an ancestral mysticete therefore remains unknown but, as discussed for birds (see above), the loss of the ability to form functional teeth could have been the consequence of a developmental shift of the oral epithelium preventing signalling molecules from reaching their mesenchymal targets.
Some comments on species lacking enamel only
Three clades of living mammals, i.e. Cingulata (armadillos) and Folivora (sloths) within Xenarthra and Tubulidentata (aardvark) within Afrotheria, are said to possess enamel-less teeth (Figs 1, 8). This claim is not correct as enamel has been described in the nine-banded armadillo.
The case of armadillos
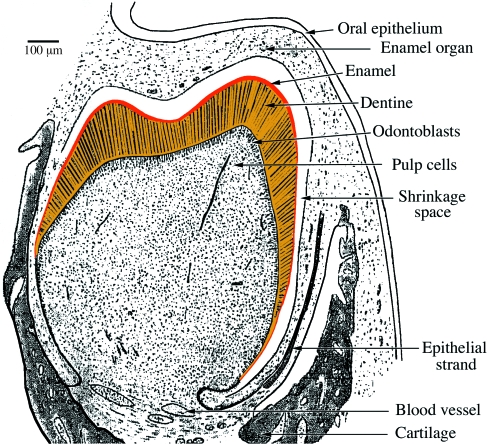
The nine-banded armadillo (Dasypus novemcinctus) has 32 teeth but giant armadillos (South America) have up to 100 teeth. Armadillos are a key group in the story of xenarthran dentition because, in addition to generalized features shared with sloths, in juveniles the teeth are covered with a thin layer of enamel, at least in D. novemcinctus, the best known species in the Cingulata lineage. The presence of enamel needs further attention as this condition is generally neglected in most text books.
Tomes (1874) was the first to describe an enamel organ in embryonic nine-banded armadillos. He believed that this enamel organ belonged to vestigial (rudimentary) teeth, as a general condition observed in several unrelated species, and that its presence was independent of enamel deposition. Nevertheless, 30 years later Spurgin (1904) reported that a thin layer of true enamel was present on the dentine surface of the first-generation teeth. This observation was confirmed by Martin (1916), who described, using a large growth series of embryos and juveniles, that not only is a thin enamel layer present in milk teeth but also that it covers permanent cheek teeth. The enamel organ is well differentiated and the four typical layers are present (inner and outer enamel epithelium, stratum intermedium and stellate reticulum). The differentiated ameloblasts are short but with well-defined Tomes’ processes. However, in permanent teeth the possibly prismatic nature of the 100-µm-thick enamel layer was not identified, if present. In both dentitions the thin enamel layer progressively disappears by abrasion.
Unfortunately there are no similar studies in the other 20 living cingulatan species. It is now agreed that the sub-family Dasypodinae (with the only genus Dasypus) is the most basal lineage of Cingulata (Delsuc et al. 2002). One can therefore speculate that the presence of a thin enamel cover was the ancestral condition in Cingulata and that enamel is either still present (but not yet described) in the three other sub-families (Euphractinae, Chlamyphorinae and Tolypeudinae) or was secondarily lost in these lineages after they separated from the Dasypodinae. This should be clarified in the future through detailed studies focusing on embryos.
In some species, the lack of enamel is compensated by a well-developed cementum, which allows a better resistance to abrasion than dentine. The cementum is thin but very hard in species of the genera Euphractus and Cabassous. In Dasypus, the cementum is not hard but it is thick, cellular and lamellar (Keil & Venema, 1963).
The presence of enamel in the nine-banded armadillo is evidence that its ancestor had typical enamel-covered crowns. Simpson (1932) found true enamel on the permanent teeth of an extinct armadillo, Utaetus buccatus, from the Eocene. The enamel layer was, however, thin and not present in all teeth due to wear. The presence of enamel in the Cingulata lineage means that this tissue was present in the common ancestor of the two Xenarthra lineages, Cingulata and Pilosa (see above). After Pilosa differentiated, enamel could have been lost either in their common ancestor [i.e. before tooth loss occurred in the Vermilingua (anteaters) lineage] or in the ancestor of Folivora (sloths) as, to our knowledge, enamel has never been reported in sloths, living or extinct (Fig. 7). Loss of enamel occurred after the acquision of a typical xenarthran tooth form.
In the nine-banded armadillo, in addition to cheek teeth, it is worthy of note that four to five tooth buds form in the anterior region of the lower jaw (probably homologous to three to four incisors and one canine). The two to three most anterior tooth germs (incisors) do not develop beyond the bud stage and degenerate before birth (Martin, 1916). The canine and sometimes the last incisor develop normally and erupt. At birth they are covered by a thin enamel layer (10–20 µm thick) (Fig. 12). In contrast to the cheek teeth they are not replaced. These descriptions support the cingulatan (and probably xenarthran) ancestor possessing a continuous dentition and the teeth being covered by enamel.
Fig. 12.
Tooth developing in the nine-banded armadillo. Note the presence of a thin layer of enamel covering the orthodentine crown. Modified after Martin (1916).
Given that previous descriptions of tooth development are from the beginning of the last century (and illustrated only with drawings) and only devoted to D. novemcinctus, new studies should be undertaken in representatives of the various armadillo sub-families, with particular attention being paid to enamel development and mineralization.
Adaptations to enamel reduction or loss in armadillos
Armadillos are generally myrmecophagous but some have a broadly omnivorous behaviour. Like anteaters, armadillos possess an elongate tongue ornamented with spiny projections and well supplied with sticky saliva that helps to catch insects. In armadillos, as in sloths (see below), teeth are hypsodont and hypselodont, and this feature compensates for their upper surface being worn after the thin enamel cover is abrased. It is also clear that the presence of a well-developed and well-mineralized cement around the crown improves the resistance to wear. These adaptations compensate for enamel being reduced or lacking (see above). The trend toward enamel reduction is characteristic of xenarthran ancestors and there is evidence that this process started (for unknown reasons) after teeth had acquired the hypsodont/hypselodont condition. It is well known that this condition was acquired independently in numerous mammalian lineages and is a convergent adaptation for highly abrasive diets, notably in herbivorous species (Jernvall & Fortelius, 2002).
Sloths
Living sloths are represented by two genera, Bradypus (four species) and Choloepus(two species), but their extinct relatives were more diverse (mylodontids, megatheriids, megalonychids and nothrotheriids) (Gaudin, 2004). They have up to 10 teeth; incisors and canines are absent but the anterior cheek tooth is triangular in cross-section and canine-like (called caniniform). It is reduced in Bradypus and separated from the rest of the cheek teeth by a short diastema. The anterior surface of the lower caniniform meets the posterior surface of the upper, wearing against each other and continuously sharpening their edges.
To our knowledge there is no information on the presence of either vestigial teeth or tooth germs in the anterior region of the jaws of sloth embryos. If we consider what occurs in other taxa lacking teeth as adults (see below), tooth germs would be expected to form in this region, to be either resorbed or lost before birth. Even the possible presence of advanced tooth germs with formation of an enamel organ could be envisaged when considering the condition existing in armadillos, their closest toothed relatives. These hypotheses should be tested in the near future and, in particular, the presence of an enamel organ and ameloblast differentiation.
Adaptations to enamel loss in sloths
The dental condition observed in living sloths can be traced back to ground sloth species that lived in the Pleistocene (2 Ma) (Bargo et al. 2006). These ancient species probably dug for food and ingested abrasive soil particles, a condition that could have played a major role in selection of dental characteristics. Living sloths feed mainly on leaves but also include some fruits, buds and even small vertebrates in their diet. As described for armadillos above, the teeth of extant and extinct sloths are hypsodont and hypselodont (Fig. 8D). This well-developed hypsodonty is considered as an evolutionary adaptation to an abrasive herbivorous diet, either as a direct (abrasive grasses) or secondary (accumulation of grit or dust on plants) consequence, and to the absence of enamel, which would make the teeth less durable and wear down faster (Vizcaíno & De Iuliis, 2003; Bargo et al. 2006). To date, our poor knowledge of tooth structure and evolution in extinct and extant sloths cannot help us to date tooth loss in this lineage.
The absence of enamel, or its extreme reduction in the Folivora ancestors, has certainly been a strong developmental constraint that has influenced the morphology of sloth dentition in such a way as to reduce the number of possible morphological responses compared with those occurring in the other placentals.
Aardvark
The aardvark, Orycteropus afer, is the only living representative of Tubulidentata and lives in sub-Saharan Africa (Shoshani et al. 1988). Due to convergent evolution with Pholidota and Xenarthra (as a result of a similar diet), Tubulidentata were long considered to be related to these clades (Edentata; e.g. Anthony, 1934). However, biochemical and molecular analyses (Dene et al. 1983; Hallström et al. 2007) have demonstrated that the aardvark is closer to elephant shrews, hyraxes, elephants, manatees and dugongs, golden moles and tenrecs. These taxa, including the aardvark, now constitute the clade Afrotheria (Fig. 8). The extant representatives of these seven afrotherian lineages differ dramatically in their morphology but this can be easily understood as the roots of each lineage extend back into the late Cretaceous or early Paleocene (75–65 Ma; Springer et al. 2003). Indeed, all afrotherian lineages had probably diverged within the first 5 million years of crown afrotherian evolution (Murphy et al. 2007). This could explain why the sister group of Tubulidentata is not well defined, although some analyses indicate a preferred relationship with Paenungulata (which include hyraxes, elephants and sea cows) (Seiffert, 2007).
The aardvark has a heterodont and diphyodont dentition. In the adult there are five permanent teeth (two pre-molars and three molars, also called cheek teeth due to a tendency for molarization of pre-molars) located toward the back of the long slender, pig-like snout (Fig. 8A). There are no incisors or canines. The tooth structure is characteristic of Tubulidentata (Anthony, 1934). As the name implies, instead of the classical pulp cavity, the teeth have numerous (around 1000) tubular pulp cavities running vertically, forming hexagonal columns (prisms) perpendicular to the occlusal plane and held together by cementum. Each prism is pierced by a tubule that does not enclose Tomes’ fibers but is homologous to the pulp cavity (Duvernoy, 1853). The teeth have no hard enamel covering and are hypselodont. Teeth are worn but the continuous growth compensates for abrasion.
In contrast to the condition observed in adults, embryos and juveniles have a complete dentition. Of the 40 tooth germs that form in the embryos, 20 are vestigial and 20 erupt. The front teeth, incisors and canines, are shed after birth and are not replaced (Lönnberg, 1906). The initial cusps of the cheek teeth are rapidly worn leaving two flat planes, with no indication of the presence of an enamel cover (Heuvelmans, 1939).
What the fossil record tells us
Afrotheria and Xenarthra are thought to have differentiated from a common ancestor during the Cretaceous and diverged approximately 100 Ma (see above). Afrotheria probably derive from primitive ungulates such as Condylarthra (Protoungulata), which lived from the late Cretaceous to the end of the Miocene. Aardvarks originated in Africa (Arambourg, 1959) and they first appear in the fossil record in the early Miocene of Kenya (20–16 Ma), a period during which the representatives of this group were more diversified and more widespread than today (fossils have been found in Europe and Asia) (Patterson, 1975). Morphologically, the genus Orycteropus has been remarkably conservative since at least the early Miocene (Pickford, 2005). All of these fossils already possess a reduced, tubulidentate dentition, which means that this structure appeared prior to the Miocene. The fossil record of Tubulidentata in older periods is scanty, however, and it is therefore difficult to date precisely when enamel was lost in this lineage (probably during the Paleocene, 60–20 Ma).
Most of the living members of the closest afrotherian lineage to Tubulidenta, Paenungulata (hyraxes, elephants and manatees and dugongs), possess two kinds of teeth, incisors and molars, separated by a diastema. The incisor in the upper jaw may be developed into a tusk that grows continuously or may be quite rudimentary. The molars erupt late in life, which is a uniting feature of the Afrotheria (Asher & Lehmann, 2008). None of these species form tubulidentate teeth or hypselodont molar teeth and enamel is present.
Adaptations to enamel loss in aardvark
Aardvark mainly feed on ants and termites, and can eat 50 000 insects in a night. They possess an elongated, flattened, protactile tongue (as long as 30 cm) that is covered by a strongly adhesive saliva that helps in catching prey (Kingdom, 1971). The continuous growth of the teeth compensates for the lack of enamel and subsequent abrasion, which is slowed down due to the presence of cementum around the tubular pulp cavities. Their strong gizzard-like stomach contains a high percentage of sand and small stones that help food digestion, as described in pangolins (see above).
The tubulidentate teeth and the absence of enamel are specific features of the lineage that led to aarvarks and it is difficult to understand how they evolved given the long period that separates the common afrotherian ancestor from the first Tubulidentata. It is agreed that the ancestor possessed a complete dentition (incisors, canines, pre-molars and molars), a condition that is strongly suggested by the milk dentition in young aardvarks. We postulate that hypsodont/hypselodont teeth appeared in an ancestral afrotherian and were positively selected for as being more adapted to resist an abrasive diet. Somewhere in the Tubulidentata lineage the ability to form enamel was lost, resulting in an increased abrasion process. This was compensated by the positive selection of tubular pulp cavities held by cementum, which are more resistant to wear. The change of diet towards ants and termites was also less abrasive and the sticky tongue allowed such a specialization.
Platypus: a species lacking teeth secondarily during ontogeny
Platypuses belong, with the echidnas, to Monotremata (see above). Ornithorhynchus anatinus is the only living representative of the Ornithorhynchidae and is only present in eastern Australia. The platypus deposits eggs similar to those of lizards and snakes, possesses a keratinized beak similar to a duck's bill, has a beaver-like tail and otter-like feet, is venomous (males), is covered by hairs and the female feeds the babies with her milk. Juvenile platypuses have three-cusped molars that are shed and substituted in adults by keratinized pads (Fig. 13A,B).
Fig. 13.
Ornithorhynchus anatinus, the platypus. (A) Lateral view of the skull in a juvenile. (B) Lateral view of the skull in adult. (C) The three teeth (a small pre-molar and two molars) on the upper left maxilla of a juvenile. (D) The three opposite teeth on the lower left jaw. Bars: A and B, 1 cm; C and D, 1 mm.
Tooth development
In platypus embryos, a dental lamina develops in the two jaws but it disappears rapidly in the most anterior region (incisor field) of the upper jaw, whereas it is retained in the lower jaw (Green, 1937). Tooth germs form as follows: five incisors, one canine, two pre-molars and three molars in the lower jaw, whereas incisors are lacking in the upper jaw. The anterior teeth do not erupt and are small and fragile but an enamel covering is present (Lester & Boyde, 1986). A single pre-molar (which is the only tooth to have a replacement) and two tribosphenic (three cusps arranged in a triangle) molars erupt (Fig. 13C,D). All erupted teeth have cusps and the dentine and enamel have the typical mammalian characters (Lester et al. 1987).
At hatching the platypus possesses both a bluntly conical os caruncle, i.e. a nodule of bone covered by a cornified epithelium (Hughes & Hall, 1998), and an egg tooth, consisting of a pulp cavity surrounded by dentine covered by an outer layer of presumptive enamel (Hill & De Beer, 1949). These tools help hatchlings to extricate themselves from the egg. The egg tooth is lost at 2 days post-hatching but the oscaruncle lasts for 11–14 weeks (Manger et al. 1998).
The three erupted teeth are lost before the young platypuses leave the breeding burrow and are not replaced. We do not know by which process these are lost in the absence of replacement teeth. Is there important remodelling of the supporting bones or is this lost related to the formation of thick heavily keratinized pads in their place (Fig. 13B)? The beak starts to form after hatching and reaches the adult size at 6 months (Manger et al. 1998). It consists of a large upper plate that arises from a cartilaginous extension of the maxillary and pre-maxillary bones, and a small lower plate originating from a cartilaginous extension of the mandibular bone.
What the fossil record tells us
Late Triassic/early Jurassic fossil remains (205 Ma) have probable monotreme affinities (Musser, 2003) but the oldest established extinct monotremes are from the early Cretaceous of Australia. They are Teinolophos trusleri (123 Ma) and Steropodon galmani (110 Ma), two steropodontids (closely related to modern ornithorhynchids), and Kallikodon richieri, a kallikodontid (100–104 Ma). Only a single molar was recovered in T trusleri but the teeth were functional as indicated by wear facets in this two-rooted molar (Rich et al. 2001). S. galmani possessed at least three functional molars and the mandibular canal suggests the presence of a beak (Archer et al. 1985; Woodburne, 2003). One pre-molar and two molars were found on the dentary of K. richieri (Flannery et al. 1995). The oldest reported ornithorhynchid isObduron insignisfrom the late Oligocene of Australia (25–15 Ma). Its close Miocene relative, O. dicksoni, had a wide flattened beak, two upper and two lower pre-molars, and two upper and three lower molars (Musser & Archer, 1998) (Fig. 7B). It is worth noting that fossil ornithorhynchids had a functional dentition in adults; the retained teeth were well developed despite the presence of a beak. During further evolution of this lineage, the tooth roots were progressively reduced and the oral epithelium took more and more importance in food processing through the formation of horny pads (Musser & Archer, 1998).
Adaptation to tooth loss in adult platypus
Platypuses are semi-aquatic animals and food is collected in the water by means of the beak. The diet consists mainly of invertebrates, the cuticle of which is broken up between the tongue and the horny grinding plates and shearing ridges on the upper and lower jaws. The edges of the beak are ornamented with sharp transverse ridges that help in catching prey. Epithelial ridges on the palate aim to secure and cut the prey in the absence of teeth. The tongue is ornamented by keratinized spines, which work against the palate to help mastication (Griffiths, 1978). All of these features are secondary adaptations that were selected during evolution as they improve food uptake and processing. They compensate for tooth loss that was, again, probably related to a relaxed functional constraint when the beak was positively selected.
Conclusion
The main question that led to us preparing this review was how to reconcile the view of a strong functional pressure acting on vertebrate teeth, on the one hand, with the existence of numerous tetrapod species lacking teeth or enamel, on the other hand. Is it so easy to lose teeth or enamel? After our comparative analysis we can answer yes, provided a pre-adapted ‘tool’ is present prior to tooth loss, otherwise tooth or enamel loss would be lethal. In fact in all tetrapod lineages examined herein, the existence of a pre-adapted tool appears to explain tooth loss (or enamel loss). Four tools were identified: beaks (birds, turtles, echidnas and platypus), elongated sticky tongues (toads, pangolins and anteaters), baleens (baleen whales) and hypsodonty/hypselodonty (armadillos, sloths and aardvark). At the beginning of the story (i.e. in a common ancestor) the tool was of secondary importance for food uptake and/or processing. However, it was certainly of some help, which explains its natural selection. Subsequently, the tool increased in importance by allowing more efficient food uptake, enhancing the ability of its owner to occupy a specific ecological niche. The positive selection of the secondary tool then relaxed the functional constraints on the teeth and they disappeared either suddenly or progressively.
To date, we have only a single explanation of what could be part of the story of tooth loss in birds: a displacement of the appropriate location of the competent (dental) epithelial cell population resulting in signalling molecules missing their target. However, tooth induction can be resurrected as has been described in the chick ta2 mutant. The mechanism responsible for this displacement is, however, unknown. Is there a mutation leading to an incorrect patterning of the oral epithelium? Such questions still remain unanswered and need to be revisited. Concerning the other beaked species (turtles and monotremes), we can only speculate that a similar mechanism was probably acting.
For the other species we could think that the relaxed pressure on teeth favoured the occurrence of various mutations. Mutations leading to tooth loss could concern a local disruption in the epithelial/mesenchymal interactions. Such a local disruption could explain loss of teeth in a particular region of the jaw only [e.g. the loss of palatine teeth in turtles, or teeth of the lower jaw in anurans and, more generally, reduction of tooth location during evolution, first in the number of bone supports concerned and then in the same bone support (Huysseune & Sire, 1998)].
Loss of enamel while conserving dentine is another interesting character that concerns Xenarthra and Tubulidentata. It appears that hypsodont/hypselodont teeth was the primary condition that occurred early in evolution in these unrelated lineages. These features are considered pre-adaptations, which allowed further adaptation to plant feeding and to an abrasive diet. They confirm the limited alternatives for tooth adaptation to an abrasive diet and an apparent facility for teeth to acquire hypsodonty/hypselodonty from a normal structure. Molars with numerous tubular pulp cavities held with cementum, open roots (hypselodonty) and lacking enamel have also been described in a basal mammal from the late Jurassic, which shows no relation to Xenarthrans (Luo & Wible, 2005). The combination of continuously growing teeth and a lack of enamel thus again goes hand in hand.
But why was enamel lost? It seems clear that such a loss was not a consequence of hypsodonty/hypselodonty as numerous, unrelated herbivorous species (e.g. some rodents and horse) have a similar adaptation but have conserved enamel. Enamel reduction was probably a first step toward complete loss of enamel but we do not know the process leading up to this reduction then loss. It is noteworthy that tooth loss (or enamel loss) is consecutive to the positive selection of a similar but convergently acquired tool (e.g. an elongated, sticky tongue) and to similar food adaptation in four unrelated lineages, i.e. feeding on small prey, ants and termites: anteaters in Xenarthra, pangolins in Pholidota, aardvark in Afrotheria and echidna in Monotremata. In addition, engulfing such prey does not require teeth but numerous secondary adaptations to help digestion, e.g. a gizzard-like stomach (pangolins and aardvark) containing small stones like in a bird's gizzard.
Eventually, although it seems common (under appropriate conditions) to lose or modify teeth (a reminiscence of an ancestral plasticity), it appears very difficult to remove the evolutionary imprints as illustrated by the presence of vestigial teeth in all toothless taxa; these rudimentary tooth germs bear witness to the ancestral condition of each species and are of considerable help for the evolutionary biologist.
Acknowledgments
We are greatly indebted to Julia Clarke for information on ancient birds, Eugene S. Gaffney (Department of Paleontology, American Museum of Natural History, New York) for information and discussion on ancient turtles, and François Pujos (Institut français des études andines, Chile) for information and the bibliography on ancient Xenarthra.
References
- Albright JT, Skobe Z. Comparative ultrastructure of oral epithelium in fish and amphibia. Arch Oral Biol. 1965;10:921–928. doi: 10.1016/0003-9969(65)90085-3. [DOI] [PubMed] [Google Scholar]
- Anderson JS, Reisz RR, Scott D, Fröbisch NB, Sumida SS. A stem batrachian from the Early Permian of Texas and the origin of frogs and salamanders. Nature. 2008;453:515–518. doi: 10.1038/nature06865. [DOI] [PubMed] [Google Scholar]
- Anderson RP, Handley CO. A new species of three-toed sloth (Mammalia: Xenarthra) from Panamá, with a review of the genus Bradypus. Proc Biol Soc Wash. 2001;114:1–33. [Google Scholar]
- Anthony R. Données nouvelles sur l’évolution de la morphologie dentaire et crânienne des Tubulidentata (Oryctéropes) Bull Soc Zool Fr. 1934;59:256–266. [Google Scholar]
- Arambourg C. Vertébrés continentaux du Miocène supérieur de l’Afrique du Nord. Paléont Mém. 1959;4:1–161. [Google Scholar]
- Archer M, Flannery TF, Ritchie A, Molnar RE. First Mesozoic mammal from Australia – an early Cretaceous monotreme. Nature. 1985;318:363–366. [Google Scholar]
- Arechaga J, Karcher-Djuricic V, Ruch JV. Neutral morphogenetic activity of epithelium in heterologous tissue recombinations. Differentiation. 1983;25:142–147. doi: 10.1111/j.1432-0436.1984.tb01349.x. [DOI] [PubMed] [Google Scholar]
- Asher RJ, Lehmann T. Dental eruption in afrotherian mammals. BMC Biol. 2008;6:14. doi: 10.1186/1741-7007-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajpai S, Gingerich PD. A new Eocene archaeocete (Mammalia, Cetacea) from India and the time of origin of whales. Proc Nat Acad Sci USA. 1998;95:15464–15468. doi: 10.1073/pnas.95.26.15464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargo S, De Iuliis G, Vizcaíno SF. Hypsodonty in Pleistocene ground sloths. Acta Palaeontol Pol. 2006;51:53–61. [Google Scholar]
- Barnes LG. Whales, dolphins and porpoises: origin and evolution of the Cetacea. Mammals. Univ Tennessee Dept Geol Sci Stud Geol. 1984;8:139–154. [Google Scholar]
- Barnes LG, Sanders AE. The transition from Archaeoceti to Mysticeti: Late Oligocene toothed mysticetes from South Carolina USA. J Vert Paleontol. 1996;16:21A. [Google Scholar]
- Bergqvist LP, Abrantes EAL, Avilla L, dos S. The Xenarthra (Mammalia) of Sao José de Itaborai Basin (upper Paleocene, Itaboraian), Rio de Janeiro, Brazil. Geodiversitas. 2004;26:323–337. [Google Scholar]
- Blanchard E. Observations sur le système dentaire chez les oiseaux. Compte-Rendus. 1860;50:540–542. [Google Scholar]
- Cannatella D, Graybeal A. Bufonidae: Tree of Life. http://tolweb.org/tree?group=Bufonidae&contgroup=Neobatrachia.
- Cannatella DC, Hillis DM. Amphibian phylogeny: phylogenetic analysis of morphology and molecules. Herpetol Monogr. 1993;7:1–7. [Google Scholar]
- Carlini AA, Scillato-Yané GJ. The oldest Megalonychidae (Xenarthra: Tardigrada); phylogenetic relationships and the amended diagnosis of the family. N Jb Geol Paläont Ab. 2004;233:423–443. [Google Scholar]
- Carlini AA, Scillato-Yané GJ, Vizcaíno SF, Dozo MT. Un singular Myrmecophagidae (Xenarthra,Vermilingua) de Edad Colhuehuapense (Oligoceno tardío, Mioceno temprano) de Patagonia, Argentina. Ameghiniana. 1992;29:176. [Google Scholar]
- Carlsson A. Ueber die Schmeizleiste bei Sterna hirundo. Anat Anz. 1896;12:72–75. [Google Scholar]
- Carroll RL, Lindsay W. Cranial anatomy of the primitive reptile, Procolophon. Can J Earth Sci. 1985;22:1571–1587. [Google Scholar]
- Caton J, Tucker A. Current knowledge in tooth development: a model mineralized system. J Anat. 2009;214:502–515. doi: 10.1111/j.1469-7580.2008.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhang Y, Jiang T-X, et al. Conservation of early odontogenic signaling pathways in Aves. Proc Nat Acad Sci USA. 2000;97:10044–10049. doi: 10.1073/pnas.160245097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappe LM. Basal bird phylogeny. Problems and solutions. In: Chiappe LM, Witmer LM, editors. Mesozoic Birds — above the Heads of Dinosaurs. Berkeley: University of California Press; 2002. pp. 448–472. [Google Scholar]
- Chiappe LM, Dyke GJ. The Mesozoic radiation of birds. Ann Rev Ecol Syst. 2002;33:91–124. [Google Scholar]
- Chiappe LM, Ji S, Ji Q, Norell MA. Anatomy and systematics of the Confuciusornithidae (Aves) from the late Mesozoic of northeastern China. Bull Am Mus Nov. 1999;242:1–89. [Google Scholar]
- Chiappe LM, Norell M, Clarke JA. A new skull of Gobipteryx minuta(Aves: Enantiornithes) from the Cretaceous of the Gobi Desert. Am Mus Novit. 2001;3333:1–15. [Google Scholar]
- Clarke JA. The morphology, phylogenetic taxonomy and systematics of Ichthyornisand Apatornis(Avialae: Ornithurae) Bull Am Mus Nat Hist. 2004;28:1–179. [Google Scholar]
- Clarke JA, Norell MA. The morphology and phylogenetic position of Apsaravis ukhaanafrom the Late Cretaceous of Mongolia. Am Mus Novit. 2002;3387:1–46. [Google Scholar]
- Clarke JA, Tambussi C, Noriega J, Erickson G, Ketcham R. First definitive fossil evidence for the extant avian radiation in the Cretaceous. Nature. 2005;433:305–308. doi: 10.1038/nature03150. [DOI] [PubMed] [Google Scholar]
- Cuvier G. Le règne animal distribué d’apres son organisation pour servir de base a l’histoire naturelle des animaux et d’introduction à l’anatomie comparée. Vol. 1. Bruxelles: Louis Haumann; 1836. pp. i–iii.pp. i–xxii.pp. 1–626. [Google Scholar]
- Dalsätt J, Zhou Z, Zhang F, Ericson Per GP. Food remains in Confuciusornis sanctussuggest a fish diet. Naturwissenschaften. 2006;93:444–446. doi: 10.1007/s00114-006-0125-y. [DOI] [PubMed] [Google Scholar]
- Davit-Béal T, Chisaka H, Delgado S, Sire J-Y. Amphibian teeth. Current knowledge, unanswered questions, and some directions for future research. Biol Rev. 2007;82:49–81. doi: 10.1111/j.1469-185X.2006.00003.x. [DOI] [PubMed] [Google Scholar]
- deBraga M, Rieppel O. Reptile phylogeny and the interrelationships of turtles. Zool J Linn Soc. 1997;120:281–354. [Google Scholar]
- De Coster PJ, Marks LA, Martens LC, Huysseune A. Dental agenesis: genetic and clinical perspectives. J Oral Pathol Med. 2009;38:1–17. doi: 10.1111/j.1600-0714.2008.00699.x. [DOI] [PubMed] [Google Scholar]
- Delsuc F, Scally M, Madsen O, et al. Molecular phylogeny of living xenarthrans and the impact of character and taxon sampling on the placental tree rooting. Mol Biol Evol. 2002;19:1656–1671. doi: 10.1093/oxfordjournals.molbev.a003989. [DOI] [PubMed] [Google Scholar]
- Delsuc F, Vizcaíno SF, Douzery EJP. Influence of Tertiary paleoenvironmental changes on the diversification of South American mammals: A relaxed molecular clock study within Xenarthrans. BMC Evol Biol. 2004;4:e11. doi: 10.1186/1471-2148-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deméré TA. Palate vascularization in an Oligocene toothed mysticete (Cetacea: Mysticeti: Aetiocetidae): implications for the evolution of baleen. In: Uhen MD, editor. IVth Triannual Convention on Evolution of Aquatic Tetrapods. Vol. 1. Lawrence: Canbrook Institute of Science, Miscellaneous Publications; 2005. p. 21. [Google Scholar]
- Deméré TA, McGowen MR, Berta A, Gatesy J. Morphological and molecular evidence for a stepwise evolutionary transition from teeth to baleen in mysticete whales. Syst Biol. 2008;57:15–37. doi: 10.1080/10635150701884632. [DOI] [PubMed] [Google Scholar]
- Dene H, Goodman M, Walz DA, Romero-Herrera AE. The phylogenetic position of aardvark (Orycteropus afer) as suggested by its myoglobin. Hoppe Seylers Z Physiol Chem. 1983;364:1585–1595. doi: 10.1515/bchm2.1983.364.2.1585. [DOI] [PubMed] [Google Scholar]
- Donoghue PCJ. Evolution of development of vertebrate teeth and scales: unravelling concepts, regulatory theories and homologies. Paleobiology. 2002;28:474–507. [Google Scholar]
- Donoghue PCJ, Sansom IJ. Origin and early evolution of vertebrate skeletonization. Microsc Res Techn. 2002;59:352–372. doi: 10.1002/jemt.10217. [DOI] [PubMed] [Google Scholar]
- Donoghue PCJ, Sansom IJ, Downs JP. Early evolution of vertebrate skeletal tissues and cellular interactions, and the canalization of skeletal development. J Exp Zool (Mol Dev Evol) 2006;306B:278–294. doi: 10.1002/jez.b.21090. [DOI] [PubMed] [Google Scholar]
- Doran GA. Palatal epithelium of a monotreme and a marsupial. Anat Anz. 1975;138:164–169. [PubMed] [Google Scholar]
- Duvernoy M. Mémoire sur les Oryctéropes du cap, du nil blanc ou d’Abyssinie et du Sénégal, et sur leurs caractères spécifiques, suivi de nouvelles recherches sur la composition microscopique de leurs dents. Ann Sci Nat Zool. 1853;19:181–203. [Google Scholar]
- Emry RJ. A North American Oligocene pangolin and other additions to the Pholidota. Bull Am Mus Nat Hist. 1970;142:456–510. [Google Scholar]
- Ernst CH, Barbour RW. Turtles of the World. Washington:: Smithsonian Institution Press; 1992. [Google Scholar]
- Estes R, Reig OA. The early fossil record of frogs: a review of the evidence. In: Vial JL, editor. Evolutionary Biology of the Anurans: Contemporary Research on Major Problems. Columbia: University of Missouri Press; 1973. pp. 11–63. [Google Scholar]
- Fabrezi M, Emerson SB. Parallelism and convergence in anuran fangs. J Zool. 2003;260:41–51. [Google Scholar]
- Ferigolo J. Evolutionary trends of the histological pattern in the teeth of Edentata (Xenarthra) Arch Oral Biol. 1985;30:71–82. doi: 10.1016/0003-9969(85)90027-5. [DOI] [PubMed] [Google Scholar]
- Flannery TF, Archer M, Rich TH, Jones R. A new family of monotremes from the Cretaceous of Australia. Nature. 1995;377:418–420. [Google Scholar]
- Ford L, Cannatella D. The major clades of frogs. Herpetol Monogr. 1993;7:94–117. [Google Scholar]
- Fordyce RE. Dental anomaly in a fossil squalodont dolphin from New Zealand and the evolution of polyodonty in whales. N Z J Zool. 1982;9:419–426. [Google Scholar]
- Fordyce RE, Barnes LG. The evolutionnary history of whales and dolphins. Ann Rev Earth Planet Sci. 1994;22:419–455. [Google Scholar]
- Fordyce RE, de Muizon C. Evolutionary history of cetaceans: a review. In: Mazin J-M, de Buffrénil V, editors. Secondary Adaptations of Tetrapods to Life in Water. Munchen: Verlag Dr Friedrich Pfeil; 2001. pp. 169–233. [Google Scholar]
- Fordyce RE, Quilty PG, Daniels J. Australodelphis morus, a bizarre new toothless ziphiid-like fossil dolphin (Cetacea: Delphinidae) from the Pliocene of Vestfold Hills, East Antarctica. Antarct Sci. 2002;14:37–54. [Google Scholar]
- Fountaine TM, Benton MJ, Dyke GJ, Nudds RL. The quality of the fossil record of Mesozoic birds. Proc Biol Sci. 2005;272:289–294. doi: 10.1098/rspb.2004.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis-West PH, Tatla T, Brickell PM. Expression patterns of the bone morphogenetic protein genes Bmp-4 and Bmp-2 in the developing chick face suggest a role in outgrowth of the primordia. Dev Dyn. 1994;201:168–178. doi: 10.1002/aja.1002010207. [DOI] [PubMed] [Google Scholar]
- Gaffney ES. The comparative osteology of the Triassic turtle Proganochelys. Bull Am Mus Nat Hist. 1990;194:1–263. [Google Scholar]
- Gaffney ES, Hutchison JH, Jenkins FA, Jr, Meeker LJ. Modern turtle origins: the oldest known Cryptodire. Science. 1987;237:289–291. doi: 10.1126/science.237.4812.289. [DOI] [PubMed] [Google Scholar]
- Gaffney ES, Meylan PA. A phylogeny of turtles. In: Benton MJ, editor. The Phylogeny and Classification of the Tetrapods. Vol. 1. Oxford: Clarendon Press; 1988. pp. 157–219. [Google Scholar]
- Gao K-Q, Chen C. A new frog (Amphibia: Anura) from the Lower Cretaceous of western Liaoning, China. Cret Res. 2004;25:761–769. [Google Scholar]
- Gardiner EG. Beitrâge zur Kenntniss des Epitrichiums und der Bildung des Vogelschnabels. Arch J Mikr Anat. 1884;24:289–338. [Google Scholar]
- Gatesy J, Hayashi C, Cronin A, Arctander P. Evidence from milk casein genes that cetaceans are close relatives of hippopotamid artiodactyls. Mol Biol Evol. 1996;13:954–963. doi: 10.1093/oxfordjournals.molbev.a025663. [DOI] [PubMed] [Google Scholar]
- Gaubert P, Antunes A. Assessing the taxonomic status of the palawan pangolin Manis culionensis(Pholidota) using discrete morphological characters. J Mammal. 2005;86:1068–1074. [Google Scholar]
- Gaudin TJ. Phylogenetic relationships among sloths (Mammalia, Xenarthra, Tardigrada): the craniodental evidence. Zool J Linn Soc. 2004;140:255–305. [Google Scholar]
- Gaudin TJ, Branham DG. The phylogeny of the Myrmecophagidae (Mammalia, Xenarthra, Vermilingua) and the relationship of Eurotamanduato the Vermilingua. J Mamm Evol. 1998;5:237–265. [Google Scholar]
- Gauthier J. Padian K, editor. Saurischian monophyly and the origin of birds. The Origin of Birds and the Evolution of Flight Mem Calif Acad Sci. 1986;8:1–55. [Google Scholar]
- Geoffroy Saint-Hilaire E. Considérations sur les pièces de la tête osseuse des animaux vertébrés, et particulièrement sur celles du crâne des oiseaux. Ann Mus Hist Nat. 1807;10:342–365. [Google Scholar]
- Geoffroy Saint-Hilaire E. Sur le système dentaire des oiseaux. Ann Gen Sci Phys. 1820;8:373–380. [Google Scholar]
- Gingerich PD. Evolutionary significance of the Mesozoic toothed birds. Smithon Contrib Paleobiol. 1975;27:23–34. [Google Scholar]
- Green HLHH. The development and morphology of the teeth of Ornithorhynchus. Phil Trans R Soc Lond B. 1937;228:367–420. [Google Scholar]
- Gregory JT. The jaws of the Cretaceous toothed birds, Ichthyornisand Hesperornis. Condor. 1952;54:73–88. [Google Scholar]
- Griffiths M. The Biology of the Monotremes. New York: Academic Press; 1978. [Google Scholar]
- Griffiths M, Wells RT, Barrie DJ. Observations on the skulls of fossil and extant echidnas (Monotremata: Tachyglossidae) Austr Mammal. 1991;14:87–101. [Google Scholar]
- Gunnel GF, Gingerich PD. Skeleton of Brachianodon westorum, a new middle Eocene metacheiromyid (Mammalia, Palaeanodonta) from the early Bridgerian (Bridger a) of the southern green river basin, Wyoming. Contrib Mus Paleont Univ Mich. 1993;28:365–392. [Google Scholar]
- Hallström BM, Kullberg M, Nilsson MA, Janke A. Phylogenomic data analyses provide evidence that Xenarthra and Afrotheria are sister groups. Mol Biol Evol. 2007;24:2059–2068. doi: 10.1093/molbev/msm136. [DOI] [PubMed] [Google Scholar]
- Harris MP, Hasso SM, Ferguson MWJ, Fallon JF. The development of archosaurian first-generation teeth in a chicken mutant. Curr Biol. 2006;16:371–377. doi: 10.1016/j.cub.2005.12.047. [DOI] [PubMed] [Google Scholar]
- Hedges SB. The origin and evolution of model organisms. Nat Rev Genet. 2002;3:838–849. doi: 10.1038/nrg929. [DOI] [PubMed] [Google Scholar]
- Hedges SB, Poling LL. A molecular phylogeny of reptiles. Science. 1999;283:998–1001. doi: 10.1126/science.283.5404.998. [DOI] [PubMed] [Google Scholar]
- Heuvelmans B. Le problème de la dentition de l’Oryctérope. Bull Mus R Hist Nat Belg. 1939;15:1–30. [Google Scholar]
- Hill JP, De Beer GR. Development of the monotrema. VII. The development and structure of the egg-tooth and the caruncle in the monotremes and on the occurrence of vestiges of the egg-tooth and caruncles in marsupials. Trans Zool Soc Lond. 1949;26:503–544. [Google Scholar]
- Hill RV. Integration of morphological data sets for phylogenetic analysis of Amniota: The importance of integumentary characters and increased taxonomic sampling. Syst Biol. 2005;54:530–547. doi: 10.1080/10635150590950326. [DOI] [PubMed] [Google Scholar]
- Hirschfeld SE. A new fossil anteater (Edentata, Mammalia) from Colombia, S.A. and evolution of the Vermilingua. J Paleontol. 1976;50:419–432. [Google Scholar]
- Hoffstetter R. Les Edentés Xénarthres, un groupe singulier de la faune néotropicale (origine, affinités, radiation adaptative, migrations et extinctions) In: Gallitelli EM, editor. Proceedings of the 1st International Meeting Paleontology, Essential of Historical Geology. Italy: S.T.E.M. Mucchi, Modena; 1982. pp. 385–443. [Google Scholar]
- Hofrichter R. Amphibian systematics. In: Hofrichter R, editor. Amphibians: The World of Frogs, Toads, Salamanders and Newts. New York: Firefly Books; 2000. pp. 36–63. [Google Scholar]
- Horovitz I, Storch G, Martin T. Ankle structure in Eocene pholidotan mammal Eomanis krebsiand its taxonomic implications. Acta Paleontol Pol. 2005;50:545–548. [Google Scholar]
- Hughes RL, Hall LS. Early development and embryology of the platypus. Philos Trans R Soc Lond B, Biol Sci. 1998;353:1101–1114. doi: 10.1098/rstb.1998.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huysseune A, Sire JY. Evolution of patterns and processes in teeth and tooth-related tissues in non mammalian vertebrates. Int J Oral Sci. 1998;106:437–481. doi: 10.1111/j.1600-0722.1998.tb02211.x. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Sire J-Y, Witten PE. Evolutionary and developmental origins of the vertebrate dentition. J Anat. 2009;214:465–476. doi: 10.1111/j.1469-7580.2009.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Amasaki H. Development and physiological degradation of tooth buds and development of rudiment of baleen plate in southern minke whale, Balaenoptera acutorostrata. J Vet Med Sci. 1995;57:665–670. doi: 10.1292/jvms.57.665. [DOI] [PubMed] [Google Scholar]
- Iwabe N, Hara Y, Kumazawa Y, et al. Sister group relationship of turtles to the bird-crocodilian clade revealed by nuclear DNA-coded proteins. Mol Biol Evol. 2005;22:810–813. doi: 10.1093/molbev/msi075. [DOI] [PubMed] [Google Scholar]
- Jernvall J, Fortelius M. Common mammals drive the evolutionary increase of hypsodonty in the Neogene. Nature. 2002;417:538–540. doi: 10.1038/417538a. [DOI] [PubMed] [Google Scholar]
- Joyce WG. Phylogenetic relationships of Mesozoic turtles. Bull Peabody Mus Nat Hist. 2007;48:3–102. [Google Scholar]
- Joyce WG, Gauthier JA. Palaeoecology of triassic stem turtles sheds new light on turtle origins. Proc Biol Sci. 2004;271:1–5. doi: 10.1098/rspb.2003.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce WG, Parham JF, Gauthier JA. Developing a protocol for the conversion of rank-based taxon names to phylogenetically defined clade names, as exemplified by turtles. J Paleontol. 2004;78:989–1013. [Google Scholar]
- Karlsen K. Development of tooth germs and adjacent structures in the whalebone whale Balaenoptera physalus. Hvalråd Skrift. 1962;45:1–56. [Google Scholar]
- Keil A, Venema B. Struktur- und Mikrohärteuntersuchungen an Zähnen von Gürteltieren (Xenarthra) Zool Beitr. 1963;9:173–195. [Google Scholar]
- Kellogg R. A new whalebone whale from the Miocene Calvert formation. Bull US Natl Mus. 1965;247:1–63. [Google Scholar]
- Kingdom J. East African Mammals: An Atlas of Evolution in Africa. New York: Academic Press; 1971. [Google Scholar]
- Kollar EJ, Fisher C. Tooth induction in chick epithelium: expression of quiescent genes for enamel synthesis. Science. 1980;207:993–995. doi: 10.1126/science.7352302. [DOI] [PubMed] [Google Scholar]
- Kordikova EG. Comparative morphology of the palate dentition in Proganochelys quenstedtiBaur 1887 from the Upper Triassic of Germany and chelonian ancestry. N Jb Geol Paläont Abh. 2002;225:195–249. [Google Scholar]
- Krause WJ, Lesson CR. The stomach of the pangolin (Manis pentadactyla) with emphasis on the pyloric teeth. Acta Anat. 1974;88:1–10. doi: 10.1159/000144218. [DOI] [PubMed] [Google Scholar]
- Laurin M, Reisz RR. A reevaluation of early amniote phylogeny. Zool J Linn Soc. 1995;113:165–223. [Google Scholar]
- Lee MSY. Pareiasaur phylogeny and the origin of turtles. Zool J Linn Soc. 1997;120:197–280. [Google Scholar]
- Lester KS, Boyde A. Scanning microscopy of platypus teeth. Anat Embryol (Berl) 1986;174:15–26. doi: 10.1007/BF00318332. [DOI] [PubMed] [Google Scholar]
- Lester KS, Boyde A, Gilkeson C, Archer M. Marsupial and monotreme enamel structure. Scann Microsc. 1987;1:401–420. [PubMed] [Google Scholar]
- Lönnberg E. On a new Orycteropus from the northern Congo, and remarks on the dentition of the Tubulidentata. Arch Zool Stockh. 1906;3:1–35. [Google Scholar]
- Loo SK, Yeo BC, Kovac H. Endothelial cells in the oral mucosa of Bufo marinus. J Anat. 1980;130:559–569. [PMC free article] [PubMed] [Google Scholar]
- Luo Z-X, Wible JR. A late Jurassic digging mammal and early mammalian diversification. Science. 2005;308:103–107. doi: 10.1126/science.1108875. [DOI] [PubMed] [Google Scholar]
- Lynch JD. Systematic status of the American leptodactylid frog genera Engystomops, Eupemphix, and Physalaemus. Copeia. 1970;3:488–496. [Google Scholar]
- Madsen O, Scally M, Douady CJ, et al. Parallel adaptive radiations in two major clades of placental mammals. Nature. 2001;409:610–614. doi: 10.1038/35054544. [DOI] [PubMed] [Google Scholar]
- Manger PR, Hall LS, Pettigrew JD. The development of the external features of the platypus (Ornithorhynchus anatinus. Philos Trans R Soc Lond B, Biol Sci. 1998;353:1115–1125. doi: 10.1098/rstb.1998.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjanovic D, Laurin M. Fossils, molecules, divergence times, and the origin of lissamphibians. Syst Biol. 2007;56:369–388. doi: 10.1080/10635150701397635. [DOI] [PubMed] [Google Scholar]
- Marsh OC. Notice of a new and remarkable fossil bird. Am J Sci. 1872;4:344. [Google Scholar]
- Marsh OC. Odontornithes, a Monograph on the Extinct Birds of North America. Washington, DC: Government Printing Office; 1880. [Google Scholar]
- Marsh OC. Birds with Teeth. United States Geological Survey, 3rd Annual Report of the Secretary of the Interior. Vol. 3. Washington, DC: Government Printing Office; 1883. pp. 43–88. [Google Scholar]
- Martin BE. Tooth development in Dasypus novemcinctus. J Morphol. 1916;27:647–691. [Google Scholar]
- Martin LD, Stewart JD. Teeth in Ichthyornis. Science. 1977;195:1331–1332. doi: 10.1126/science.195.4284.1331. [DOI] [PubMed] [Google Scholar]
- Martin LD, Stewart JD, Whetstone KN. The origin of birds: structure of the tarsus and teeth. Auk. 1980;97:86–93. [Google Scholar]
- Matalova E, Fleischmannova J, Sharpe PT, Tucker AS. Tooth agenesis: from molecular genetics to molecular dentistry. J Dent Res. 2008;87:617–623. doi: 10.1177/154405910808700715. [DOI] [PubMed] [Google Scholar]
- McGeoch DJ, Gatherer D. Integrating reptilian herpesviruses into the family Herpesviridae. J Virol. 2005;79:725–731. doi: 10.1128/JVI.79.2.725-731.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna MC, Bell SK. Classification of Mammals Above the Species Level. New York: Columbia University Press; 1997. [Google Scholar]
- McKenna MC, Wyss AR, Flynn JJ. Paleogene pseudoglyptodont xenarthrans from central Chile and Argentine Patagonia. Am Mus Novit. 2006;3536:1–18. [Google Scholar]
- Mikkola ML, Thesleff I. Ectodysplasin signaling in development. Cytokine Growth Factor Rev. 2003;14:211–224. doi: 10.1016/s1359-6101(03)00020-0. [DOI] [PubMed] [Google Scholar]
- Mitsiadis TA, Chéraud Y, Sharpe P, Fontaine-Pérus J. Development of teeth in chick embryos after mouse neural crest transplantations. Proc Nat Acad Sci USA. 2003;100:6541–6545. doi: 10.1073/pnas.1137104100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller-Krull M, Delsuc F, Churakov G, et al. Retroposed elements and their flanking regions resolve the evolutionary history of xenarthran mammals (armadillos, anteaters, and sloths) Mol Biol Evol. 2007;24:2573–2582. doi: 10.1093/molbev/msm201. [DOI] [PubMed] [Google Scholar]
- Murphy WJ, Eizirik E, O’Brien SJ, et al. Resolution of the early placental mammal radiation using Bayesian phylogenetics. Science. 2001;294:2348–2351. doi: 10.1126/science.1067179. [DOI] [PubMed] [Google Scholar]
- Murphy WJ, Pringle TH, Crider TA, Springer MS, Miller W. Using genomic data to unravel the root of the placental mammal phylogeny. Genome Res. 2007;17:413–421. doi: 10.1101/gr.5918807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PF. A unique jaw mechanism in the echidna, Tachyglossus aculeatus(Monotremata) Austr J Zool. 1981;29:1–5. [Google Scholar]
- Musser AM. Review of the monotreme fossil record and comparison of palaeontological and molecular data. Comp Biochem Physiol A, Mol Integr Physiol. 2003;136:927–942. doi: 10.1016/s1095-6433(03)00275-7. [DOI] [PubMed] [Google Scholar]
- Musser AM, Archer M. New information about the skull and dentary of the Miocene platypus Obdurodon dicksoni, and a discussion of ornithorhynchid relationships. Philos Trans R Soc Lond B, Biol Sci. 1998;353:1063–1079. doi: 10.1098/rstb.1998.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido M, Rooney AP, Okada N. Phylogenetic relationships among cetartiodactyls based on insertions of short and long interpersed elements: hippopotamuses are the closest extant relatives of whales. Proc Natl Acad Sci USA. 1999;96:10261–10266. doi: 10.1073/pnas.96.18.10261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev S, Montoya-Burgos JI, Margulies EH, et al. Early history of mammals is elucidated with the ENCODE Multiple Species Sequencing Data. PLoS Genet. 2007;3:e2. doi: 10.1371/journal.pgen.0030002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisa C, Kitamura N, Sasaki M, et al. Immunohistochemical study on the distribution and relative frequency of endocrine cells in the stomach of the Malayan pangolin, Manis javanica. Anat Histol Embryol. 2005;34:373–378. doi: 10.1111/j.1439-0264.2005.00626.x. [DOI] [PubMed] [Google Scholar]
- Novacek MJ. Fossils, topologies, missing data, and the higher level phylogeny of eutherian mammals. Syst Biol. 1992a;41:58–73. [Google Scholar]
- Novacek MJ. Mammalian phylogeny: shaking the tree. Nature. 1992b;356:121–125. doi: 10.1038/356121a0. [DOI] [PubMed] [Google Scholar]
- Novacek MJ, Wyss AR. Higher-level relationships of the recent eutherian orders: morphological evidence. Cladistics. 1986;2:257–287. doi: 10.1111/j.1096-0031.1986.tb00463.x. [DOI] [PubMed] [Google Scholar]
- Nowak RM. Walker's Mammals of the World. 6th edn. I. Baltimore: Johns Hopkins University Press; 1999. and II. [Google Scholar]
- Ostrom JH. Archaeopteryxand the origin of birds. Biol J Linn Soc. 1976;8:91–812. [Google Scholar]
- Pascual R, Archer M, Juareguizar EO, et al. First discovery of monotremes in South America. Nature. 1992;356:704–706. [Google Scholar]
- Patterson B. The fossil aardvarks (Mammalia: Tubulidentata) Bull Mus Comp Zool. 1975;147:185–237. [Google Scholar]
- Patterson B, Pascual R. The fossil mammal fauna of South America. In: Keast A, Erk FC, Glass BP, editors. Evolution of Mammals and Southern Continents. Albany, NY: State University of New York Press; 1972. pp. 247–309. [Google Scholar]
- Peyer B. Comparative Odontology. Chicago: The University Chicago Press; 1968. [Google Scholar]
- Pickford M. Orycteropus(Tubulidentata, Mammalia) from Langebaanweg and Baard's Quarry, Early Pliocene of South Africa. CR Palevol. 2005;4:715–726. [Google Scholar]
- Pujos F, De Iuliis G. Late Oligocene Megatherioidea fauna (Mammalia: Xenarthra) from Salla-Luribay (Bolivia): new data on basal sloth radiation and Cingulata-Tardigrada split. J Vert Paleontol. 2007;27:132–144. [Google Scholar]
- Pujos F, De Iuliis G, Argot C, Werdelin L. A peculiar climbing Megalonychidae from the Pleistocene of Peru and its implication for sloth history. Zool J Linn Soc. 2007;149:179–235. [Google Scholar]
- Reif WE. Evolution of dermal skeleton and dentition in vertebrates. The odontode regulation theory. Evol Biol. 1982;15:278–386. [Google Scholar]
- Rich TH, Vickers-Rich P, Trusler P, et al. Monotreme nature of the Australian Early Cretaceous mammal Teinolophos. Acta Palaeontol Pol. 2001;46:113–118. [Google Scholar]
- Rieppel O. Turtle origins. Science. 1999;283:945–946. doi: 10.1126/science.283.5404.945. [DOI] [PubMed] [Google Scholar]
- Rieppel O, deBraga M. Turtles as diapsid reptiles. Nature. 1996;384:453–455. [Google Scholar]
- Röse C. Ueber die rudimentäre Zahnanlagen der Gattung Manis. Anat Anz. 1892;7:748–758. [Google Scholar]
- Rose KD, Eberle JJ, McKenna MC. Arcticanodon dawsonae, a primitive new palaeanodont from the lower Eocene of Ellesmere Island, Canadian High Arctic. Can J Earth Sci. 2004;41:757–763. [Google Scholar]
- Rougier GW, de la Fuente MS, Arcucci AB. Late Triassic turtles from South America. Science. 1995;268:855–858. doi: 10.1126/science.268.5212.855. [DOI] [PubMed] [Google Scholar]
- Rowe T, Rich TH, Vickers-Rich P, Springer M, Woodburne MO. The oldest platypus and its bearing on divergence timing of the platypus and echidna clades. Proc Natl Acad Sci USA. 2008;105:1238–1242. doi: 10.1073/pnas.0706385105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander PM. Teeth and jaws. In: Padian K, editor. Encyclopedia of Dinosaurs. New York: Academic Press; 1997. pp. 717–725. [Google Scholar]
- Scally M, Madsen O, Douady CJ, de Jong WW, Stanhope MJ, Springer MS. Molecular evidence for the major clades of placental mammals. J Mamm Evol. 2001;8:239–277. doi: 10.1038/35054544. [DOI] [PubMed] [Google Scholar]
- Scheyer TM, Sander PM. Shell bone histology indicates terrestrial palaeoecology of basal turtles. Proc Biol Sci. 2007;274:1885–1893. doi: 10.1098/rspb.2007.0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scillato-Yané GJ. Sobre un Dasypodidae (Mammalia, Xenarthra) de edad Riochiquense (Paleoceno Superior) de Itaborai, Brasil. An Acad Bras Cienc. 1976;48:527–530. [Google Scholar]
- Secord R, Gingerich PD, Bloch JI. Mylanodon rosei,a new metacheiromyid (Mammalia, Palaeanodonta) from the late Tiffanian (Late Paleocene) of northwestern Wyoming. Contrib Mus Paleontol Univ Mich. 2002;30:385–399. [Google Scholar]
- Seiffert ER. A new estimate of afrotherian phylogeny based on simultaneous analysis of genomic, morphogical, and fossil evidence. BMC Evol Biol. 2007;7:224. doi: 10.1186/1471-2148-7-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JP, Ellis SA. A scanning electron microscope study of the odontoids and teeth in Hemiphractus proboscideus(Anura: Hylidae) J Zool. 1989;219:533–544. [Google Scholar]
- Shoshani J, Goldman CA, Thewissen JGM. Orycteropus afer. Mamm Spec. 1988;300:1–8. [Google Scholar]
- Shubin NH, Jenkins FA., Jr An Early Jurassic jumping frog. Nature. 1995;377:49–52. [Google Scholar]
- Sibley C, Ahlquist JE. Phylogeny and Classification of Birds. New Haven: Yale University Press; 1990. [Google Scholar]
- Simpson GG. Enamel on the teeth of an Eocene edentate. Am Mus Novit. 1932;567:1–4. [Google Scholar]
- Simpson GG, Granger W, Wortman JL. Metacheiromysand the Edentata. Bull Am Mus Nat Hist. 1931;59:295–381. [Google Scholar]
- Sire J-Y, Davit-Béal T, Delgado S, Van der heyden C, Huysseune A. The first generation teeth in non-mammalian lineages: evidence for a conserved ancestral character? Microsc Res Techn. 2002;59:408–434. doi: 10.1002/jemt.10220. [DOI] [PubMed] [Google Scholar]
- Sire J-Y, Delgado S, Girondot M. Hen's teeth with enamel cap: from dream to impossibility. BMC Evol Biol. 2008;8:246. doi: 10.1186/1471-2148-8-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AB, Peterson KJ. Dating the time of origin of major clades: molecular clocks and the fossil record. A Rev Earth Planet Sci. 2002;30:65–88. [Google Scholar]
- Smith MM. Vertebrate dentitions at the origin of jaws: when and how pattern evolved. Evol Dev. 2003;5:394–413. doi: 10.1046/j.1525-142x.2003.03047.x. [DOI] [PubMed] [Google Scholar]
- Springer MS, Murphy WJ, Eizirik E, O’Brien SJ. Placental mammal diversification and the Cretaceous-Tertiary boundary. Proc Natl Acad Sci USA. 2003;100:1056–1061. doi: 10.1073/pnas.0334222100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurgin AM. Enamel in the teeth of an embryo edentate (Dasypus novemcinctusLinn.) Am J Anat. 1904;3:75–84. [Google Scholar]
- Thewissen JG, Cooper LN, Clementz MT, Bajpai S, Tiwari BN. Whales originated from aquatic artiodactyls in the Eocene epoch of India. Nature. 2007;450:1190–1194. doi: 10.1038/nature06343. [DOI] [PubMed] [Google Scholar]
- Tims HWM. Tooth vestiges and associated mouth parts in the Manidae. J Anat Physiol. 1908;42:375–387. [PMC free article] [PubMed] [Google Scholar]
- Tomes CS. On the existence of an enamel organ in the armadillo. Quart J Microsc Sci. 1874;48:44–48. [Google Scholar]
- Trueb L. Bones, frogs and evolution. In: Vial JL, editor. Evolutionary Biology of the Anurans: Contemporary Research on Major Problems. Columbia, Missouri: University of Missouri Press; 1973. pp. 65–132. [Google Scholar]
- Uhen MD. Evolution of dental morphology (cetacean) In: Perrin WF, Würsig B, Thewissen JGM, editors. Encyclopedia of Marine Mammals. San Diego: Academic Press; 2002. pp. 316–319. [Google Scholar]
- Uhen MD. Evolution of marine mammals: back to the sea after 300 millions years. Anat Rec. 2007;290:514–522. doi: 10.1002/ar.20545. [DOI] [PubMed] [Google Scholar]
- Utrecht WLV. On the growth of the baleen plate of the fin whale and the blue whale. Bijdr Dierk. 1965;35:3–38. [Google Scholar]
- Van Dissel-Scherft MC, Vervoort WD. Development of the teeth in fetal Balaenoptera physalus(Cetacea, mystacoceti) Proc Kon Ned Akad Wet. 1954;57:196–210. [Google Scholar]
- van Rheede T, Bastiaans T, Boone DN, et al. The platypus is in its place: nuclear genes and indels confirm the sister group relation of monotremes and therians. Mol Biol Evol. 2006;23:587–597. doi: 10.1093/molbev/msj064. [DOI] [PubMed] [Google Scholar]
- Van Tuinen M, Dyke GJ. Calibration of galliform molecular clocks using multiple fossils and genetic partitions. Mol Phylogenet Evol. 2004;30:74–86. doi: 10.1016/s1055-7903(03)00164-7. [DOI] [PubMed] [Google Scholar]
- Vizcaíno SF. Identificacion especifica de las ‘mulitas’, género Dasypus(Mammalia, Dasypodidae), del noroeste argentino. Descripcion de una nueva especie. Mastozool Neotrop. 1995;2:5–13. [Google Scholar]
- Vizcaíno SF, De Iuliis G. Evidence for advanced carnivory in fossil armadillos (Mammalia; Xenarthra: Dasypodidae) Paleobiology. 2003;29:123–138. [Google Scholar]
- Vizcaíno SF, Loughry WJ. The Biology of the Xenarthra. Gainesville: University Press of Florida; 2008. [Google Scholar]
- Vizcaíno SF, Scillato-Yané GJ. An Eocene tardigrade (Mammalia, Xenarthra) from Seymour Island, West Antarctica. Antarct Sci. 1995;7:407–408. [Google Scholar]
- Wang YH, Upholt WB, Sharpe PT, Kollar EJ, Mina M. Odontogenic epithelium induces similar molecular responses in chick and mouse mandibular mesenchyme. Dev Dyn. 1998;213:386–397. doi: 10.1002/(SICI)1097-0177(199812)213:4<386::AID-AJA4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Westerman M, Edwards D. DNA hybridization and the phylogeny of monotremes. In: Augee ML, editor. Platypus and Echidnas. Sydney: Royal Zoological Society of New South Wales; 1992. pp. 28–34. [Google Scholar]
- Whetstone KN, Martin LD. New look at the origin of birds and crocodiles. Nature. 1979;279:234–236. doi: 10.1038/279234a0. [DOI] [PubMed] [Google Scholar]
- Woodburne MO. Monotremes as pretribosphenic mammals. J Mamm Evol. 2003;10:195–248. [Google Scholar]
- Wu P, Jiang TX, Suksaweang S, Widelitz RB, Chuong CM. Molecular shaping of the beak. Science. 2004;305:1466–1467. doi: 10.1126/science.1098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Jiang T-X, Shen J-Y, Widelitz RB, Chuong C-M. Morphoregulation of avian beaks: Comparative mapping of growth zone activities and morphological evolution. Dev Dyn. 2006;235:1400–1412. doi: 10.1002/dvdy.20825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Zhou H, Chen Y-Q, Liu Y-F, Qu L-H. Mitogenomic perspectives on the origin and phylogeny of living amphibians. Syst Biol. 2005;54:391–400. doi: 10.1080/10635150590945278. [DOI] [PubMed] [Google Scholar]
- Zhou Z. The origin and early evolution of birds: discoveries, disputes, and perspectives from fossil evidence. Naturwissenschaften. 2004;91:455–471. doi: 10.1007/s00114-004-0570-4. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Zhang F. Jeholornis compared to Archaeopteryx, with a new understanding of the earliest avian evolution. Naturwissenschaften. 2003;90:220–225. doi: 10.1007/s00114-003-0416-5. [DOI] [PubMed] [Google Scholar]
- Zweers GA, Vanden berge JC, Berkhoudt H. Evolutionary patterns of avian trophic diversification. Zoology. 1997;100:25–57. [Google Scholar]