Table 2.
Thermal [4 + 2] Cycloaddions of of Allenamides.
| entry | allenamidesa | time [h] | cycloadducts | yield [%]b |
|---|---|---|---|---|
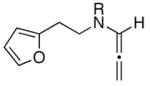 |
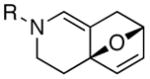 |
|||
| 1 | 13a: R = p-Ns | 12 | 14a: R = p-Ns | 92 |
| 2 | 13b: R = Boc | 20 | 14b: R = Boc | 65 |
| 3 | 13c: R = (−)-menthyl | 20 | 14c: R = (−)-menthyl | 54c |
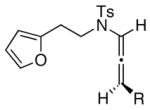 |
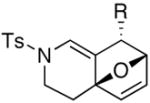 |
|||
| 4 | (±)-15a: R = Ph | 2 | 16a: R = Ph | 77d |
| 5 | (±)-15b: R = Me | 30 | 16b: R = Me | 57d |
| 6 |
17 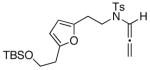
|
<12 |
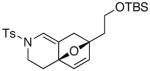 19 19
|
77 |
| 7 |
18 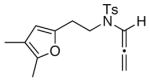
|
4 |
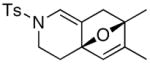 20 20
|
93 |
| 8 |
21 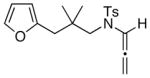
|
24 |
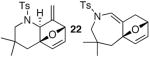 23 23
|
95e |
| 9 |
24 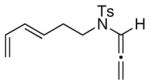
|
20 |
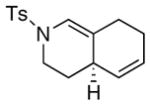 25 25
|
78 |
Unless otherwise noted, all reactions were carried out in THF at 85 °C at concn = 0.10 M. Reactions in entries 3 and 8 were run in toluene. Entries 4 and 8 were run at 45 °C and 110 °C, respectively.
Isolated yields.
Only one isomer by 1H NMR but absolute configuration unassigned.
16a and 16b were found as a ~ 3:1 inseparable isomeric mixture.
Regioisomeric ratio of regioisomers 22 and 23 is ~ 4:1.
