Table 3.
Tandem Isomerization–[4 + 2] Cycloadditions.
| entry | propargyl amidesa | time [h] | cycloadducts | yield [%]b |
|---|---|---|---|---|
| 1 |
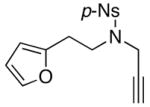 30 30
|
24 |
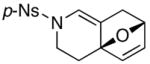 14a 14a
|
84 |
| 2 |
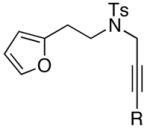 |
24 |
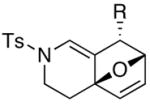 |
79c |
| 3 | 24 | 42c,e | ||
| 4 | 16 | 25d,e | ||
| 5 |
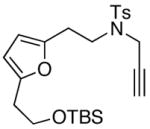 32 32
|
14 |
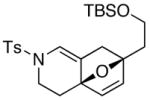 19 19
|
63 |
Unless otherwise noted, all reactions were carried out in THF at concn = 0.10 M with 20 mol % of t-BuOK. For entries 1 and 5: Reaction temp = 65 °C; for entries 3 and 4: temp = 85 °C; and for entry 2: temp = 25 °C.
Isolated yields.
dr = ~3:1.
dr = ~2:1.
The reaction was slower, and also observed was hydrolysis of the starting allenamide.
