Table 1.
Optimization of thiazolidine exchange reaction of 3a.
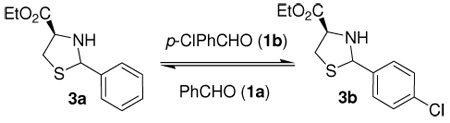 | ||||
|---|---|---|---|---|
| entry | Reaction conditionsa | time (h) | 3a/3b ratiob | 2 (%)c |
| 1 | pH 4, rt | 24 | 45/55 | 3 |
| 2 | pH 4, rt | 48 | 44/56 | 2 |
| 3 | pH 4, rt | 72 | 46/54 | 10 |
| 4 | pH 5, rt | 24 | 71/29 | 5 |
| 5 | pH 5, rt | 48 | 68/32 | 10 |
| 6 | pH 5, rt | 72 | 52/48 | 9 |
| 7 | pH 6, rt | 24 | 96/4 | 0 |
| 8 | pH 6, rt | 48 | 97/3 | 0 |
| 9 | pH 6, rt | 72 | 90/10 | 0 |
| 10 | pH 7, rt | 72 | 98/2 | 0 |
| 11 | pH 4, 35 °C | 24 | 45/55 | 18d |
| 12 | pH 4, 35 °C | 48 | 35/65 | 16e |
The starting concentration of each component was 1 mM; the reaction mixture was stirred in a buffered acetate solution at pH 4 and pH 5, and in a phosphate solution at pH 6 and pH 7.
The ratio was determined by 1H NMR.
The mass balance was quantitative
Total yield 3a + 3b (64%).
Total yield 3a + 3b (49%).
