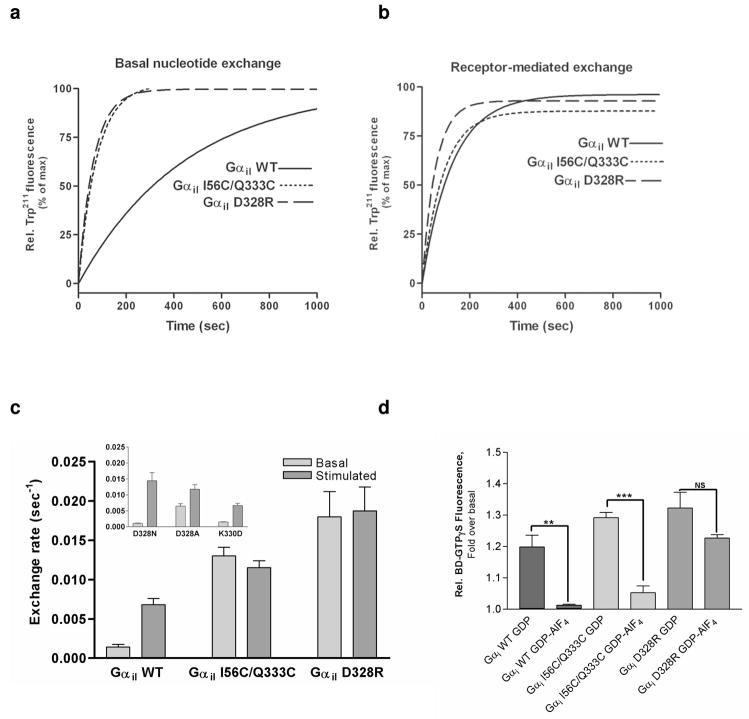
Figure 2.
Elevated rates of basal nucleotide exchange in Gαi1 mutants. (a) Basal Exchange. The basal rate of GTP-γS binding was determined by monitoring the relative increase in the intrinsic Trp211 fluorescence. Basal exchange for the wild-type is shown with a solid line (bottom trace), basal exchange for the I56C/Q333C double mutant is shown with a small-dashed line (------), and basal exchange for the D328R mutant is shown with a large-dashed line (——). (b) Receptor catalyzed nucleotide exchange. Nucleotide exchange for the receptor-catalyzed wild-type and mutant Gαi1 subunits was determined by monitoring the relative increase in the intrinsic Trp211 fluorescence performed in presence of 500 nM rhodopsin. The addition of rhodopsin does not significantly affect the rate of nucleotide exchange in the I56C/Q333C or D328R Gαi1 subunits but catalyzes a 4-fold rate enhancement for wild-type Gαi1 subunits (p=0.0005). (c) Quantitation of the initial rates of basal and rhodopsin-catalyzed nucleotide exchange monitored by the relative increase in the intrinsic Trp211 fluorescence for wild-type and mutant Gαi1 subunits. (d) Basal nucleotide exchange of GDP- or GDP-AlF4−-bound Gαi1 subunits as measured by the increase in emission from BODIPY-labeled GTPγS upon addition of GDP-bound Gαi1 or GDP-AlF4−-bound Gαi1 subunits. Relative increase was plotted as percentage of basal BODIPY-GTPγS fluorescence prior to addition of Gα subunits. Data are the average of three independent experiments (**p = 0.0027 and p=0.0001).