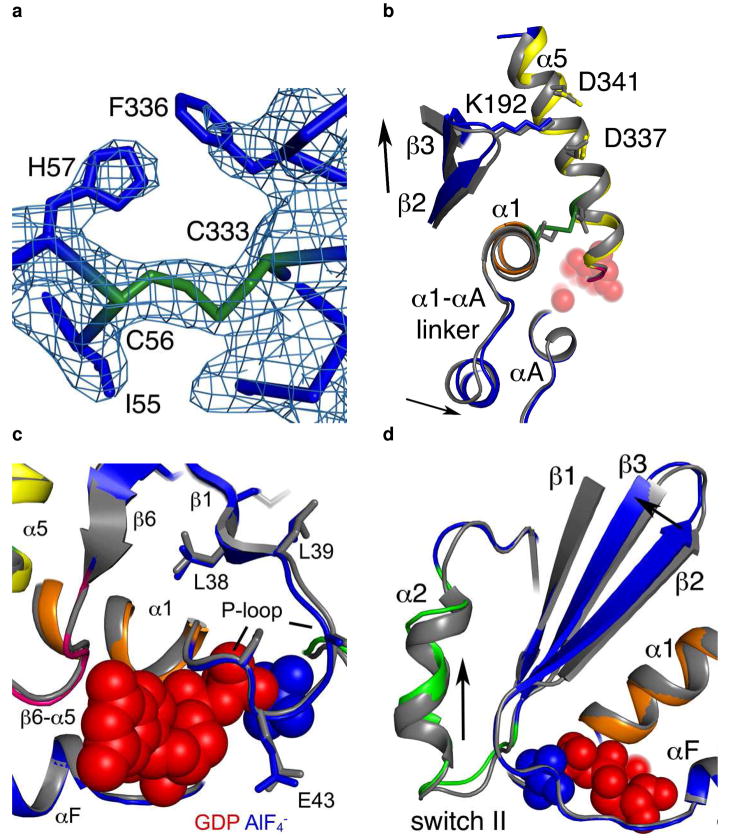
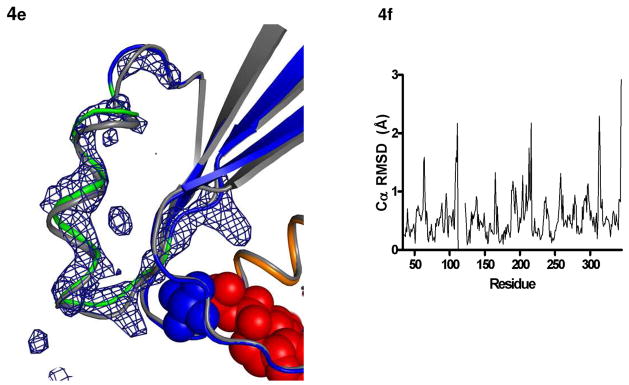
Figure 4.
Structural changes in the I56C/Q333C Gαi1. All comparisons are made between the I56C/Q333C Gαi1 subunit and the wild-type Gαi1 subunit crystallized in complex with GDP-AlF4−. Coloring for panels (b) - (d) is the same as in Figure 1. (a) 2|Fo| - Fc| density (blue mesh) contoured at 1.2 σ shows a connection between the side chains at positions 56 and 333 and is consistent with the formation of a disulfide bond. (b) Movement of the α5 helix, the β2-β3 hairpin and the α1-αA linker that connects the GTPase (dark blue) and helical domains (cyan). (c) Movement of the P-loop. (d) Movement of the β2-β3 hairpin away from the α1 helix toward the β1 strand. The hairpin directly connects the second linker (residues 177–182) and switch II (residues 202–215; light green). (e) |Fo| - |Fc| simulated annealing omit density calculated using CNS and contoured at 3.0 σ after the removal of all atoms between residues 202–217 (α2 helix and switch II) highlights the shift between the I56C/Q333C (blue/green) and wild type (grey) Gαi1. The GDP molecule is in red and the AlF4− is in blue. (f) RMSD of each alpha carbon atom in I56C/Q333C Gαil structure as compared to wild-type Gαil (PDB 1GFI, selected regions overlaid in 4a-e).