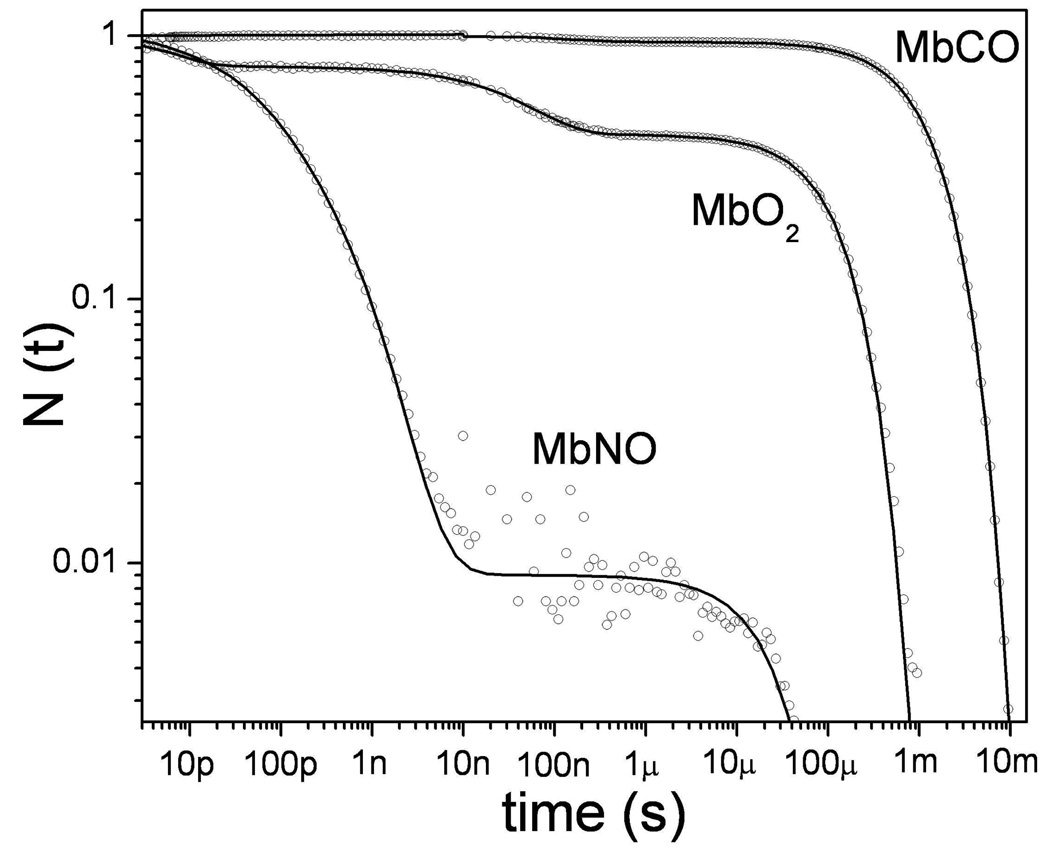
Figure 3.
The rebinding kinetics of diatomic ligands to ferrous myoglobin, following photolysis at room temperature in aqueous solution, are displayed on a logarithmic scale. The survival probablility, N(t), measures the population of the deoxyMb photoproduct as ligand rebinding proceeds. The decay times for the three ligands span approximately 9 decades in time. In the case of CO, approximately 96% of the photodissociated CO molecules escape from the heme pocket into the solvent so that rebinding primarily involves a relatively slow bimolecular process. In contrast, 99% of the photolyzed NO molecules rebind geminately in two separable phases with time constants of 15ps and 170 ps. In the case of O2 there are again two geminate phases, but the second phase (~50ns) is well separated from the first (~6ps). The bimolecular yield for oxygen is in the range 40–50%.