Table 1.
Asymmetric Epoxidation of α-Isopropylstyrene with Ketones 3a
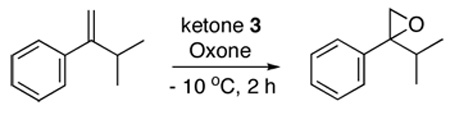 | ||||
|---|---|---|---|---|
| entry | ketone | solvent | conv. (%)b | ee (%)b |
| 1 | 3d | CH3CN/DMM (1/2) | 97 | 71 |
| 2 | DME | 94 | 81 | |
| 3 | DME/n-BuOH | 99 | 76 | |
| 4 | 1,4-dioxane | 94 | 84 | |
| 5 | 1,4-dioxane/DME (2/1) | 99 | 80 | |
| 6 | 1,4-dioxane/n-BuOH (1/1) | 100 | 78 | |
| 7 | 3a | 91 | 82 | |
| 8 | 3b | 69 | 71 | |
| 9 | 3c | 10 | nd | |
| 10 | 3e | 100 | 83 | |
| 11 | 3f | 98 | 83 | |
| 12 | 3g | 80 | 52 | |
| 13 | 3h | 99 | 84 | |
All epoxidations were carried out with the olefin (0.2 mmol), ketone 3 (0.06 mmol), Oxone (0.32 mmol), and K2CO3 (1.344 mmol) in organic solvent (3 mL) and buffer (0.1 M K2CO3/AcOH, pH 9.3; 2 mL) at −10 °C for 2 h.
The conversion and ee were determined by chiral GC (B-DM column).
