Table 2.
Asymmetric Epoxidation of 1,1-Disubstituted Terminal Olefins with Ketone 3da
| entry | substrate | yield (%)b | ee (%) | config.e |
|---|---|---|---|---|
| 1 | R = Me | 60 | 62c | (+)−(S)13a |
| 2 | R = Et | 71 | 78d | (+)–(S)13a |
| 3 | R = n-Pr | 90 | 75d | (+) |
| 4 | R = i-Bu | 54 | 74d | (+) |
| 5 | R = c-C6H11 | 62 | 77c | (+) |
| 6 | R = t-Bu | 43 | 86d | (+) |
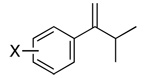 |
||||
| 7 | X = H | 71 | 84d | (+) |
| 8 | X = p-i-Pr | 51 | 82c | (+) |
| 9 | X = p-MeO | 94 | 84c | (+) |
| 10 | X = p-F | 78 | 74d | (+) |
| 11 | X = p-Br | 68 | 78d | (+) |
| 12 | X = m-Me | 57 | 82c | (+) |
| 13 | X = m-F | 74 | 81d | (+) |
| 14 | X = o-F | 72 | 88d | (+) |
| 15 | 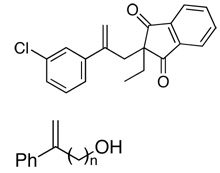 |
51 | 66c | (−)–(S)13b |
| 16 | n = 1 | 93 | 77c | (+)–(R)13c |
| 17 | n = 2 | 47 | 72c | (+) |
| 18 | n = 3 | 62 | 74c | (+) |
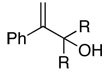 |
||||
| 19 | R = Me | 76 | 87c | (+)–(S)13a |
| 20 | R = Et | 85 | 87d | (+) |
| 21 | R,R = (CH2)4 | 86 | 88d | (+)–(S)13a |
| 22 | 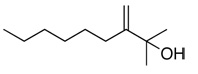 |
78 | 60d | (+) |
All epoxidations were carried out with the olefin (0.2 mmol), ketone 3d (0.06 mmol), Oxone (0.32 mmol), and K2CO3 (1.344 mmol) in 1,4-dioxane (3 mL), and buffer (0.1 M K2CO3/AcOH, pH 9.3; 2 mL) at −10 °C for 2 h (4 h for entries 6, 11, 13, and 14).
Isolated yield except entry 7 which is crude yield.
The ee was determined by chiral HPLC (Chiracel OD column).
The ee was determined by chiral GC (B-DM column).
The absolute configurations were determined by comparing the measured optical rotations and HPLC trace with reported ones.
