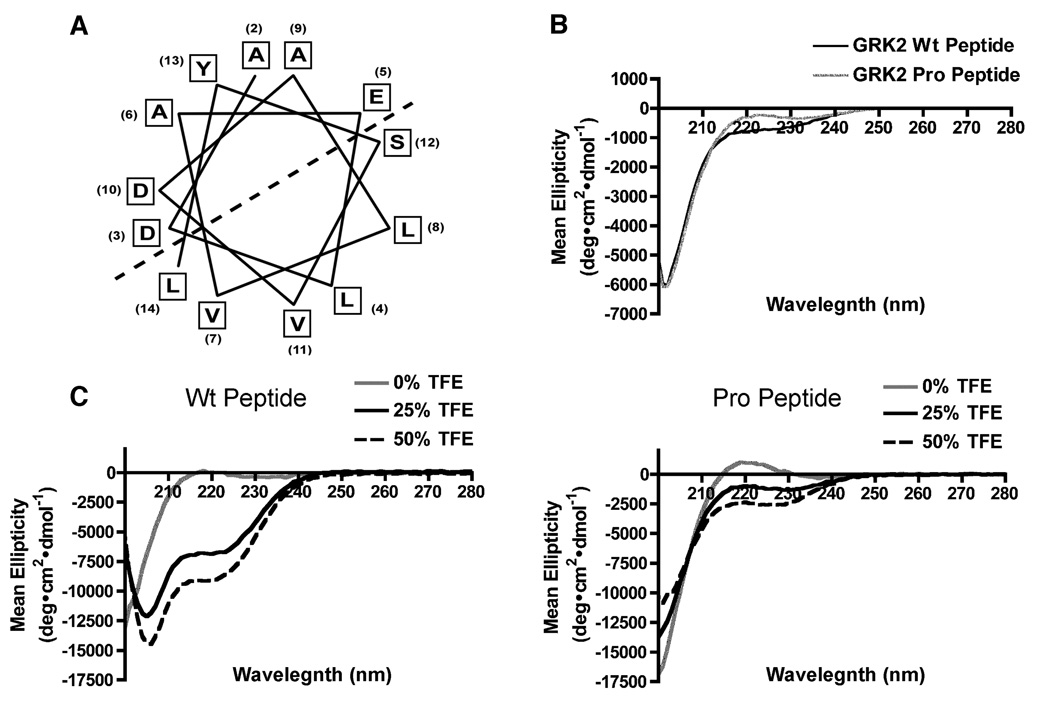
Figure 3. The first 14 amino acids form an amphipathic alpha helix.
(A) Secondary structure prediction programs revealed that the first 14 amino acids of GRK2 form an amphipathic alpha helix, where one face is rich in polar residues and the other rich in hydrophobic residues as delineated by the dashed line. (B) Representative CD spectra of the GRK2 Wt (black line) and Pro (grey line) amino terminal peptides were determined in the range of 200–280 nm at 4°C using a spectrapolarimeter. (C) Increasing concentrations of the monohydric alcohol TFE increased the helicity of the GRK2 peptide. 0% TFE shown in gray represents a random coil, 25% TFE (solid black) has ~20% helicity and at 50% TFE (dashed black), there is ~44% helicity.