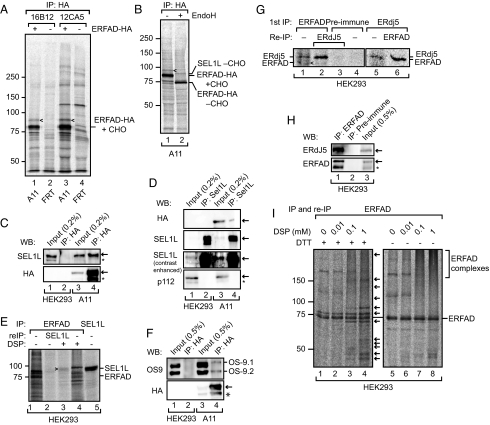
Fig. 2.
ERFAD interacts with the ERAD components SEL1L, OS-9, and ERdj5. (A) Immunoprecipitation of ERFAD-HA. Cells stably expressing ERFAD-HA (A11) and control cells (FRT) were [35S] pulse-labeled for 16 h, Triton X-100 lysates immunoprecipitated with anti-HA (16B12 and 12CA5), and samples separated by reducing SDS/PAGE. The position of a coimmunoprecipitating 90 kDa band is indicated by arrowheads. (B) Immunoprecipitation of ERFAD-HA with 16B12, undigested (lane 1) and digested (lane 2) with EndoH. The position of the protein identified by mass spectrometry—SEL1L—is indicated. CHO, N-glycans. (C) SEL1L coimmunoprecipitates with ERFAD-HA. Immunoprecipitations from lysates of A11 or HEK293 cells were performed with anti-HA, and subsequently analyzed by Western blotting as indicated. *, background band. (D) ERFAD-HA coimmunoprecipitates with SEL1L. Immunoprecipitations from lysates of A11 cells were performed with anti-SEL1L and subsequently analyzed by Western blotting with anti-HA, anti-SEL1L (contrast-enhanced blot included to better see the input), and anti-p112 as a specificity control. *, background band. (E) Endogenous ERFAD and SEL1L coimmunoprecipitate upon cross-linking with DSP. After incubation with DSP, ERFAD was immunoprecipitated from [35S] pulse-labeled HEK293 cells. The immunoprecipitate was either analyzed directly (lanes 1 and 4) or reimmunoprecipitated with antibodies against SEL1L (lane 2 and 3). For comparison an anti-SEL1L immunoprecipitate is loaded in lane 5. Samples were analyzed by reducing SDS/PAGE, which resolves the thiol-cleavable cross-link between ERFAD and SEL1L. (F) OS-9.1 and 9.2 coprecipitate with ERFAD-HA. Immunoprecipitations from A11 or HEK293 cell lysates were performed with anti-HA, and analyzed by Western blotting with antibodies against OS-9 and the HA tag. *, background band. (G) Endogenous ERFAD and ERdj5 coimmunoprecipitate. ERFAD was immunoprecipitated from [35S] pulse-labeled HEK293 cells with anti-ERFAD (SG2480). The immunoprecipitate was either analyzed directly (lane 1) or reimmunoprecipitated with anti-ERdj5 (lane 2). As a control, preimmune serum was used instead of anti-ERFAD (lanes 3 and 4). In another experiment, ERdj5 was immunoprecipitated from pulse-labeled HEK293 cells and either analyzed directly (lane 5) or immunoprecipitated with anti-ERFAD (lane 6). Samples were analyzed by reducing SDS/PAGE. Arrowhead, ERFAD. (H) Endogenous ERFAD and ERdj5 coimmunoprecipitate. Immunoprecipitations from lysates of HEK293 cells were performed with anti-ERFAD (1F6) or preimmune serum and subsequently analyzed by Western blotting with anti-ERdj5 and anti-ERFAD. *, background band. (I) Numerous proteins immunoprecipitate with endogenous ERFAD upon DSP cross-linking. Immunoprecipitates of ERFAD after treatment with increasing concentrations of DSP were either analyzed under reducing or nonreducing conditions. Arrows indicate proteins that coprecipitate with ERFAD upon cross-linking. For complete audiographs see Fig. S3.