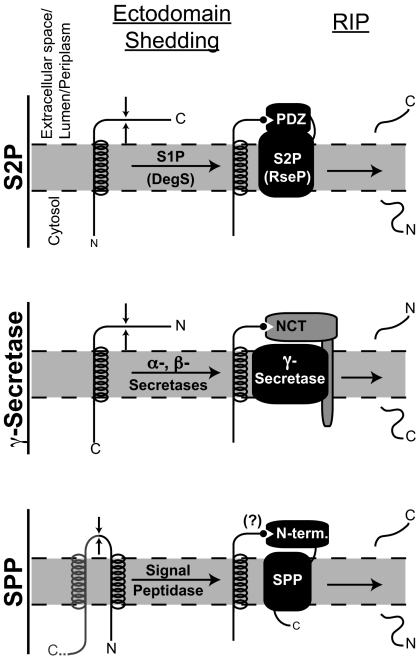
Fig. 1.
A common mechanism for the role of ectodomain shedding in Rip? Li et al. (3) report that upon ectodomain shedding of RseA by the site-1 protease DegS, the newly exposed C-terminal-most amino acid Val-148 binds one of the PDZ domains of RseP to facilitate the regulated intramembrane proteolytic (Rip) cleavage by the S2P RseP (Top). Such a mechanism is consistent with studies of γ-secretase-mediated Rip cleavage of the amyloid precursor protein and other substrates (Middle): upon ectodomain shedding by α- or β-secretase, the newly generated N terminus of the substrate binds to the NCT subunit of γ-secretase (11). A third family of I-CLiPs is typified by SPP, whose cleavage of substrates, like that of S2P and γ-secretase substrates, is also preceded by ectodomain shedding (Bottom). The N-terminal portion of SPP is not part of the catalytic core of SPP and may therefore function analogously to the PDZ domain of S2Ps and NCT of γ-secretase.