Figure 6.
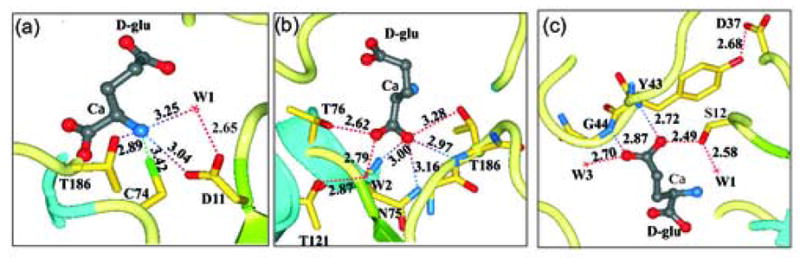
Interactions of amino acids within the RacE2 catalytic site with D-glutamate. (a) Potential interactions between the active site residues and the α amino group of D-glutamate. (b) Interactions involved in recognition and stabilization of the Cα-carboxyl group. (c) Interactions likely involved in substrate/product recognition and stabilization of the D-glutamate side chain carboxyl group. A highly conserved loop forms the floor of the active site with D37 forming a hydrogen bond with Y43 that is significant and results in orienting this loop in a conformation that favors substrate binding thereby maintaining an important secondary structure conformation that is involved in substrate recognition. The dashed lines in all panels represent distances between atoms in Å.
