Abstract
Tristetraprolin is a vertebrate CCCH tandem zinc finger protein that can bind to and destabilize certain mRNAs containing AU-rich element binding sites. zfs1 is the single gene in the fission yeast, Schizosaccharomyces pombe, that encodes a protein containing the critical features of the tristetraprolin zinc finger domain. zfs1 has been linked to pheromone signal transduction control and to the coordination of mitosis, but no biological function has been ascribed to the zfs1 protein. Through a functional genomics approach we compared transcript levels in wild-type and zfs1-deficient S. pombe strains; those elevated in the zfs1-deficient strain were examined for the presence of potential tristetraprolin-like binding sites. One such potential target transcript was encoded by arz1, a gene encoding a protein of unknown function that contains armadillo repeats. arz1 mRNA decay was inhibited in the zfs1-deficient strain when it was expressed under the control of a thiamine-repressible promoter. Mutations within one AU-rich element present in the arz1 3′-untranslated region protected this transcript from zfs1-promoted decay, whereas mutating another potential binding site had no effect. Binding assays confirmed a direct interaction between zfs1 and arz1 mRNA-based probes; this interaction was eliminated when key residues were mutated in either zfs1 zinc finger. zfs1 and its targets in S. pombe represent a useful model system for studies of zinc finger protein/AU-rich element interactions that result in mRNA decay.
Tristetraprolin (TTP)2 is a well characterized CCCH tandem zinc finger (TZF) domain RNA-binding protein that can regulate the decay of many mRNAs (1–6). The presence of a conserved TZF domain of this type defines a small family of proteins in man that consists of TTP, ZFP36L1 (7), and ZFP36L2 (8). Interactions between the TZF domain and type II AU-rich elements (AREs) found in the 3′-untranslated regions (UTRs) of target mRNAs have been the focus of multiple investigations (9–14). These have included the use of a synthetic TTP TZF domain peptide to examine specificity of ARE target variants (9, 11, 12), the evaluation of target mRNA decay as it is influenced by mutated TZF domains expressed in co-transfected cells (14), cell-free deadenylation of ARE-containing mRNAs (10, 15), and the use of engineered stable transcripts to assess ARE specificity, as measured through mRNA decay (10). The solution structure of the TZF domain of ZFP36L2 (TIS11D) bound to the UUAUUUAUU nonamer has been solved (13), providing insight into the interaction between the TZF domain and a target ARE. A core UAUUUAU heptamer unit appears to be necessary for optimal binding affinity of the TZF domain for an ARE (10, 14).
Examination of the genome of the fission yeast Schizosaccharomyces pombe revealed only one member of the TTP family, encoded by the zfs1 gene, which has a TZF domain at its C terminus (16). zfs1 is necessary for coordinating septum formation with exit from mitosis (17). zfs1-deficient cells exhibit a reduced rate of cell division, with decreased cell size, and do not respond properly to pheromone (16, 17). Pheromone insensitivity can be overcome by overexpression of proteins that control the upstream portion of the pheromone signaling cascade (16). To date no specific biochemical function of the zfs1 protein has been demonstrated, but intact zinc fingers are necessary for the biological roles of zfs1 described previously (16). No evidence of DNA or RNA binding has been presented for zfs1.
In contrast to S. pombe, Saccharomyces cerevisiae expresses two members of the TTP family, CTH1 and CTH2 (18, 19). Unlike the effects observed with disruption of zfs1 in S. pombe, disruption of either CTH1 or CTH2 had no apparent effect on growth (18). Overexpression of CTH1 or mouse TTP, but not CTH2, in S. cerevisiae reduced growth rates by 50% (18), and increased zfs1 expression in S. pombe resulted in a comparable level of growth reduction after 24 h. CTH2 is important for regulating mRNA stability in response to iron depletion (19). Recent data indicate that zfs1 is not a functional homologue of CTH2 with respect to iron sensitivity (20), and S. pombe and S. cerevisiae differ substantially in their mechanisms of iron metabolism (19–21).
In a microarray study designed to identify transcripts elevated in a zfs1-deficient strain, we identified a previously uncharacterized gene that we have named arz1 (for armadillo-repeat containing zfs1 target 1). We hypothesized that, like TTP and its mammalian family members, zfs1 might function as an ARE-binding, mRNA-destabilizing protein. We show that zfs1 can bind specifically to AREs within the arz1 mRNA 3′-UTR in a zinc finger-dependent fashion, and stimulate the rate of arz1 mRNA decay in intact cells. These data suggest that this system holds promise for more detailed biochemical investigations of the mechanisms of action of this protein family, as well as the physiological function of zfs1 in this organism.
Experimental Procedures
Yeast Strains
A S. pombe zfs1-deficient strain was used in a direct comparison with a representative wild-type strain, which was originally used as the background cell type for zfs1 gene targeting (17). These strains were gifts from Dr. Viesturs Simanis (École Polytechnique Fédérale De Lausanne, Lausanne, Switzerland). Strains were grown at 30 °C in Edinburgh minimal medium (EMM) supplemented with 50 mg/liter of adenine, histidine, leucine, lysine, and uracil each (EMM+5S) or with SP minus Leu (EMM+4S-Leu) when transformed (media and supplements were purchased from Q-biogene, Morgan, CA).
Steady-state Comparison of S. pombe Strains by Microarray
S. pombe wild-type and zfs1-deficient strains were grown to 1.0 A600 in EMM+5S at 30 °C for steady-state analysis. Total cellular RNA was prepared from four replicate cultures. For these and all other cultures in this study, cells from 1.5 ml of culture were centrifuged at 16,100 × g for 1 min at room temperature, and total RNA was harvested from the cell pellet after storage at −80 °C using the MasterPure yeast RNA purification kit (Epicenter, Madison, WI). Gene expression analysis was conducted using Yeast Genome 2.0 GeneChip® arrays (Affymetrix, Santa Clara, CA) containing both S. cerevisiae and S. pombe probe sets. RNA (1 μg) was amplified using the Affymetrix One-Cycle cDNA Synthesis protocol. For each array, 5 μg of amplified biotin-cRNAs was fragmented and hybridized to the array for 16 h at 45 °C in a rotating hybridization oven using the Affymetrix Eukaryotic Target Hybridization Controls and protocol. Slides were stained with streptavidin/phycoerythrin using a double-antibody staining procedure and washed utilizing the Mini_euk2v3 protocol of the Affymetrix Fluidics Station FS450 for antibody amplification. Arrays were scanned with an Affymetrix Scanner 3000, and data were obtained using the GeneChip® operating software. The resulting files (.dat, .cel, and .chp) were imported into the Rosetta Resolver system, and the S. cerevisiae probe sets were masked out prior to data preprocessing and error modeling (22). The data are accessible at the NCBI Gene Expression Omnibus (GEO), as described under “Results,” and selected transcripts elevated in the zfs1-deficient strain that contain potential binding sites are presented in Table 1.
Table 1. Expressed genes elevated in zfs1-deficient S. pombe that contain putative 3′-UTR AREs.
| S. pombe gene | Description | Statusa | Fold changeb | p value |
|---|---|---|---|---|
| SPCC1494.03 | Armadillo-repeat containing | Hypothetical | 4.30204 | <0.00E-45 |
| SPBC20F10.03 | Conserved eukaryotic | Hypothetical | 3.81255 | <0.00E-45 |
| SPAC343.06c | Phospholipid scramblase | Homology | 2.95178 | <0.00E-45 |
| SPAC8C9.16c | Mitochondrial oxidation resistance protein | Homology | 2.75289 | 3.20E-26 |
| SPAC4D7.10c | Histone acetyltransferase subunit | Homology | 2.38142 | 3.36E-24 |
| SPAC7D4.12c | Hypothetical protein | Hypothetical | 2.29411 | 3.99E-24 |
| SPAC30D11.07 | Nht1 DNA endonuclease III | Experimental | 2.18523 | 5.97E-18 |
| SPBC365.09c | CCHH zinc finger/conserved hypothetical | Homology | 1.90405 | 7.45E-11 |
| SPAC31A2.12 | Arrestin family | Homology | 1.84812 | 9.49E-27 |
| SPAC19G12.15c | Tpp1 (trehalose-6-phosphate phosphatase) | Experimental | 1.82499 | 8.57E-26 |
| SPBC17D11.01 | Nep1 (nedd8 protease) | Experimental | 1.75315 | <0.00E-45 |
| SPBC1289.13c | Galactosyltransferase | Homology | 1.67609 | 3.70E-31 |
Indicates the status of the characterization of these genes listed in the S. pombe gene database. “Experimental” indicates some level of experimental characterization, “Homology” indicates a description based on homology to known proteins of other species, and “Hypothetical” indicates a predicted protein that has not been assigned a specific description by homology.
Reflects increased transcript levels in the zfs1-deficient strain relative to wild type. This comparison is based on the means of four separate experiments in each case.
nmt Transformants
The coding region of SPCC1494.03 (arz1) plus 1 kb of downstream sequence predicted to include the 3′-UTR was PCR-amplified using primers F1 and R1 (all PCR primers are listed in Table 2), and the product was cloned into a TOPO TA vector system based on the nmt expression system (nmt41) (Invitrogen) (23). The nmt41 vector is known to express constructs at ∼15% of the level of the nmt1 vector (16, 23, 24). Site-directed mutagenesis was carried out using the QuikChange system (Stratagene), and primers listed in Table 3, to produce the following nmt/arz1 mutant constructs: MuA, which has two A to G mutations; MuB, which has two A to G mutations for each of two nonamers (four total); B1 and B2, which have two A to C mutations at each of the AREb nonamers separately, and CCC, which has three mutations of TAA to CCC. Mutations were confirmed in purified nmt/arz1 plasmids by dideoxy-chain terminator-based sequencing (25). Both S. pombe strains described above were transformed with the un-mutated nmt/arz1 plasmid to produce the W1 and Z1 reference transformant strains, and the mutated nmt/arz1 plasmids were transformed into the wild-type S. pombe to produce MuA, MuB, B1, B2, and CCC transformant strains for direct comparison with W1 and Z1 reference strains following previously described methods (26, 27). Positive transformants were screened for retained plasmids by PCR using a 5′ nmt vector specific primer, F2, and a 3′ arz1 internal primer, R2 (Table 2).
Table 2. PCR primers used in this study.
| Primer | Sequence (5′ → 3′) | Gene target |
NCBI accession number |
Target sequence position |
Product length |
|---|---|---|---|---|---|
| bp | |||||
| F1 | ATGACCGCTTCTGATACAGTCTAC | arz1 | NC_003421.2 | 2,325,172-2,325,196 | 2479 |
| R1 | TTCGCAACGCTGGAAAGTTTGTGT | 2,327,651-2,327,627 | |||
| F2 | TTTCAATCTCATTCTCACTTTCTGA | nmt/arz1 | pREP (23) | 1,098-1,122 | 374 |
| R2 | GGGCTCGATACTCTCGTGAATCC | NM_001023516.1 | 325-303 | ||
| F3 | CTCTTGAACTCCTTAGGG | arz1 | NM_001023516.1 | 98-115 | 665 |
| R3 | TGCTATTAGAGTCGTCGG | 763-746 | |||
| F4 | TCCCTTCTTCAGTCATGCCC | zfs1 | NM_001022372.1 | 251-270 | 460 |
| R4 | ACAGAGTTGCGGATGTTTGTC | 711-690 | |||
| F5 | TTGTTGACTGAGGCTCCTTTGAAC | actin | NM_001021513.1 | 310-333 | 801 |
| R5 | AAACGATACCCAGGTCCGCTCTC | 1,111-1,090 | |||
| F6a | ATGAGAATATTGGTACCCTGTTTCAGGGTCCGGTTTATTCTCCTATGTCTCG | zfs1 | NM_001022372.1 | 4-13 | 1212 |
| R6a | CTCGATTAACTAGTAGCTAGCTCAAGGAGATTGCTTAATAGTTG | 1,193-1,215 |
Underlined bases indicate zfs1 specific primer regions, whereas italic text highlights SspI and SpeI restriction sites for cloning into restriction enzyme-digested expression vector.
Table 3. Mutagenesis primers used in this study.
| Primera | Sequence (5′ → 3′)b | Gene target | NCBI accession number | Target sequence position |
|---|---|---|---|---|
| FMuA | CAGCGTTATTGCTAATTGTTTGTTTTTTCG | arz1 | NC_003421.2 | 2,326,720-2,326,749 |
| RMuA | AGGAATAATCGAAAAAACAAACAATTAGC | 2,326,758-2,326,730 | ||
| FMuB | CAAGTTTCATCCTTGTTTGTTACCATTAATTGTTTGTTTATCTATC | arz1 | NC_003421.2 | 2,326,853-2,326,898 |
| RMuB | AGTTGTAGATAGATAAACAAACAATTAATGGTAACAAACAAGGATG | 2,326,905-2,326,860 | ||
| FB1 | CTCAATTACAAGTTTCATCCTTCTTTCTTACC | arz1 | NC_003421.2 | 2,326,845-2,326,876 |
| RB1 | AAATAATTAATGGTAAGAAAGAAGGATG | 2,326,887-2,326,860 | ||
| FB2 | TTTATTACCATTAATTCTTTCTTTATC | arz1 | NC_003421.2 | 2,326,868-2,326,894 |
| RB2 | GAGTTGTAGATAGATAAAGAAAGAATTAATG | 2,326,906-2,326,876 | ||
| FCCC | TTCATCCTTATTTATTACCATCCCTTATTTATTTATCTATC | arz1 | NC_003421.2 | 2,326,858-2,326,898 |
| RCCC | ATGGAGTTGTAGATAGATAAATAAATAAGGGATGG | 2,326,909-2,326,876 | ||
| Fzfs1c | TACAAAAGCGAGCGTGGCCGATCTTTCATG | zfs1 | NM_001022372.1 | 1,093-1,123 |
| Rzfs1c | ACCATACATCATGAAAGATCGGCCACGCTCGC | 1,131-1,099 | ||
| Fzfs1h | CTCTAAATGTCAATTTGCGATTGGCAACCAGG | zfs1 | NM_001022372.1 | 1,032-1,063 |
| Rzfs1h | TTCAGTTCCTGGTTGCCAATCGCAAATTGAC | 1,070-1,040 |
The first five sets of primers were used for arz1 ARE mutations; the last two sets of primers were used for mutating zfs1 TZF domain conserved residues, Cys-270 and His-351, to glycine and isoleucine, respectively.
Bold residues in the sequence indicate a mutated base.
S. pombe Iron Depletion
Wild-type S. pombe was grown in EMM+5S from ∼0.1 A600 to log phase at 30 °C (reached ∼1.0 A600 at 20 h) in the presence (treatment) or absence (control) of 750 μm ferrozine (Sigma), an iron chelator, following an approach used for S. cerevisiae (19). 5 μg of total cellular RNA was used for Northern analysis (see below) of zfs1 transcript levels.
Northern Analyses
arz1-, zfs1-, and actin-specific probes were generated by PCR using gene-specific primers (Table 2), and 50 ng of each probe fragment was used for random-primed labeling with a High Prime kit (Roche Applied Science) to incorporate [α-32P]dCTP (PerkinElmer Life Sciences). The actin control was used to examine loading consistency, in combination with gel staining using acridine orange, and for quantitative correction during PhosphorImager densitometric evaluation (ImageQuaNT 5.1, Molecular Dynamics, GE Healthcare, Piscataway, NJ).
S. pombe wild-type and zfs1-deficient strains were grown to 1.0 A600 in EMM+5S at 30 °C for steady-state analysis. A total of 5 μg of total cellular RNA was used in each gel lane for all Northern analyses, following standard methods (28). W1 and Z1 transformant strains were grown to 1.0 A600 in 30 ml of EMM+4S-Leu. A time “0” sample (1.5 ml of culture) was first collected, and the cell suspension was centrifuged at 16,100 × g for 1 min at room temperature, after which the pellet was frozen in liquid N2. Thiamine (10 μm final concentration) was then added to the cultures. These were then sampled such that pellets were frozen at 10, 20, and 40 min after thiamine addition. A second screen focused on 4-min time points (4, 8, 12, 16, and 20 min) was carried out in an effort to determine the half-life of the arz1 mRNA in the W1 and Z1 transformant strains. Cell pellets of transformants were used for total cellular RNA isolation and Northern analysis. Endogenous mRNA control samples were evaluated in direct comparison with each set of transformant strain comparisons and used for correction during quantification of nmt/arz1-expressed mRNA.
Total cellular RNA from W1, MuA, MuB, B1, B2, or CCC mutant nmt/arz1 constructs was compared directly to RNA from Z1 by Northern analysis. Direct comparisons of the four samples were performed following the same protocol used for the initial W1 and Z1 initial time-course comparisons. Northern blots were washed extensively with two final washes at 60 °C in 1× SSC, 0.1% SDS before exposure to film or phosphorimaging screens. Blots were exposed to phosphorimaging screens for 2–3 h, and these were scanned using the Typhoon system (Molecular Dynamics, GE Healthcare) and analyzed using ImageQuaNT and GraphPad (San Diego, CA) Prism, version 4. All graphs show paired comparisons using Students t test (for direct comparison of individual means) or one-way analysis of variance (used for comparisons of multiple means), and error bars indicate standard deviations.
zfs1 Protein Production and RNA Gel Shift Assays
The zfs1 coding region was PCR-amplified using primers F6 and R6 (Table 2), containing SspI and SpeI restriction sites, respectively, for cloning into restriction enzyme-digested expression vector. A PCR-derived restriction digest fragment was cloned into an expression vector LacZ promoter upstream of the maltose-binding protein (MBP) coding sequence (cytoplasmic) along with a tetracycline resistance cassette (AstraZeneca, Wilmington, DE). The expression construct was transformed into Escherichia coli BL21(DE3) containing a TF16 (trigger factor) expression construct and a chloramphenicol resistance cassette. A clone bearing both expression constructs was grown in selective medium at 37 °C to 0.4 A600. Expression of TF16 was induced by the addition of 1 ml of 0.5 g/ml l-arabinose for 1 h, followed by induction and expression of the MBP/zfs1 construct with 0.5 mm isopropyl β-d-1-thiogalactopyranoside for 20 h at 18 °C (shaking at 200 rpm). Cultures were chilled on ice, and cells were pelleted by centrifugation at 5,000 × g for 20 min at 4 °C and then lysed in 40 ml of cold Buffer A (20 mm Tris-HCl, pH 7.4, 200 mm NaCl, 0.1% Triton X-100) by ultrasonication. The cellular debris was pelleted by centrifugation at 10,000 × g for 20 min at 4 °C. The supernatant was then applied to 5 g of amylose resin (New England Biolabs, Ipswich, MA) that had been washed with Buffer A in preparation for batch method purification in a 50-ml polypropylene tube, following the manufacturer's protocol. Approximately 30 mg of protein was obtained (5–6 mg/ml) from the amylose resin. Protein concentrations were assessed using the BCA protein assay (Pierce) and were confirmed by SDS-PAGE, using albumin as a standard, and purity was estimated at >90% by SDS-PAGE Coomassie Blue staining. Site-directed mutants of the MBP/zfs1 construct were made (29), using primer pairs Fzfs1C and Rzfs1C, and Fzfs1H and Fzfs1H (Table 3), that would change Cys-370 to Gly (C370G) and His-351 to Ile (H351I), respectively, using the amino acid numbering system in NCBI accession number CAB75997.1, which we predicted would eliminate RNA binding based on previous studies in mammalian family members (3) and homology modeling presented in Fig. 5. Recombinant mutant proteins were expressed and purified using the same strategy that was used for the wild-type MBP/zfs1 protein and for MBP alone. The C370G and H351I amino acid mutant proteins yielded ∼24 mg and 18 mg of total protein, respectively.
Figure 5. Predicted zfs1 TZF domain interaction with an ARE.
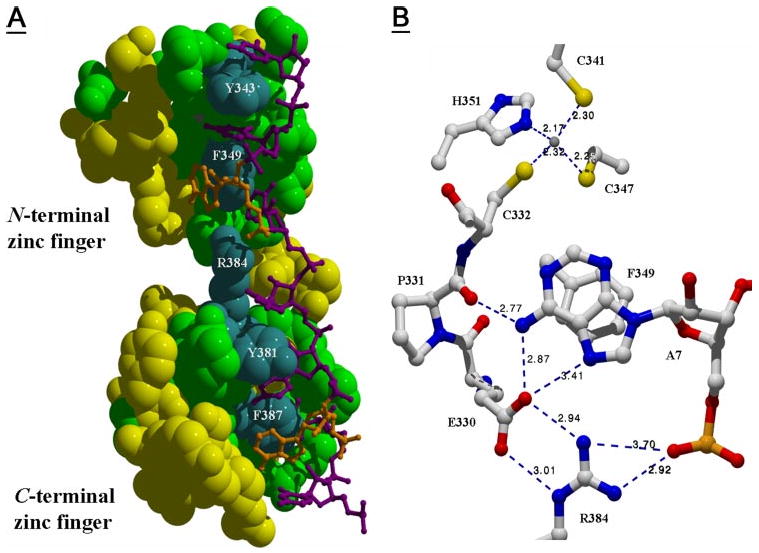
A, shown is the predicted structure of the zfs1 TZF domain complexed with UUAUUUAUU. Amino acids identical to human TTP are highlighted in green or blue, and represent primarily the ARE binding residues (variable residues are yellow). Select conserved residues (white text labels) include the aromatic residues known to form stacking interactions (13) and the Arg-384 residue of the C-terminal zinc finger. B, shows selected residues from the zfs1 TZF domain and their potential interactions with A7 of the UUAUUUAUU nonamer in proximity to the zinc binding core. This picture illustrates the possible interactions between residues of the N-terminal zinc-finger, Arg-384 from the C-terminal zinc-finger and the A7 nucleotide. Distances between functional group core atoms are shown.
Single-stranded RNA oligonucleotides were obtained from Invitrogen that represented the arz1 AREa and AREb sequences, as well as binding site mutants of each of these sequences (Table 4). A human tumor necrosis factor-α ARE-based oligonucleotide of comparable length was also tested in gel shift assays (Table 4). Each oligonucleotide was end-labeled with [32P]pCp at the 3′-end using T4 RNA ligase (New England Biolabs, Ipswich, MA). Approximately 100 fmol of each probe (1 × 104 cpm) was used per reaction in binding experiments.
Table 4. RNA oligonucleotides used in this study.
| Probe | Sequence (5′ → 3′)a | NCBI accession number | Length |
|---|---|---|---|
| b | |||
| TNFα | UUAUUUAUUAUUUAUUUAUUAUUUAUU | AB103618.1 | 27 |
| AREa | UAUUGCUAAUUAUUUAUUUUUUCGAUUA | NC_003421.2 | 28 |
| AREa mutant | UAUUGCUAAUUCUUUCUUUUUUCGAUUA | NC_003421.2 | 28 |
| AREb | CUUAUUUAUUACCAUUAAUUAUUUAUUU | NC_003421.2 | 28 |
| B1 mutant | CUUCUUUCUUACCAUUAAUUAUUUAUUU | NC_003421.2 | 28 |
| B2 mutant | CUUAUUUAUUACCAUUAAUUCUUUCUUU | NC_003421.2 | 28 |
| AREb mutant | CUUCUUUCUUACCAUUAAUUCUUUCUUU | NC_003421.2 | 28 |
Bold bases indicate those considered mutated compared to the normal arz1 seqeunces (AREa and AREb).
Approximately 4.25 μg of each recombinant MBP/zfs1 protein (50 pmol) was used in RNA electrophoretic mobility shift assays in comparison with 4.25 μg of MBP alone (∼100 pmol). In each case, the designated amount of probe was preincubated with binding buffer (10 mm HEPES (pH 7.6), 40 mm KCl, 5% (v/v) glycerol, 50 μg of heparin, 3 mm MgCl2, 1.2 μg of yeast tRNA) for 20 min. An aliquot of each protein was then combined with each probe separately, along with 2 units of Ribonuclease Inhibitor (Invitrogen), and the mixture was incubated at room temperature for 20 min before gel loading. The reactions were separated on 8% polyacrylamide gels in 0.4× TBE (40 mm Tris-HCl (pH 8.3), 40 mm boric acid, and 8 mm EDTA) running buffer for 1.5 h at 250 V. Gels were then dried at 80 °C for 30 min on absorbent paper and exposed to film.
mRNA Structure Prediction
MFOLD (3.1.2), software for prediction of RNA secondary structure by Energy Minimization (30), was used to analyze the AREb sequence (Fig. 2F).
Figure 2. Mutations at arz1 AREb influence zfs1-dependent arz1 mRNA instability.
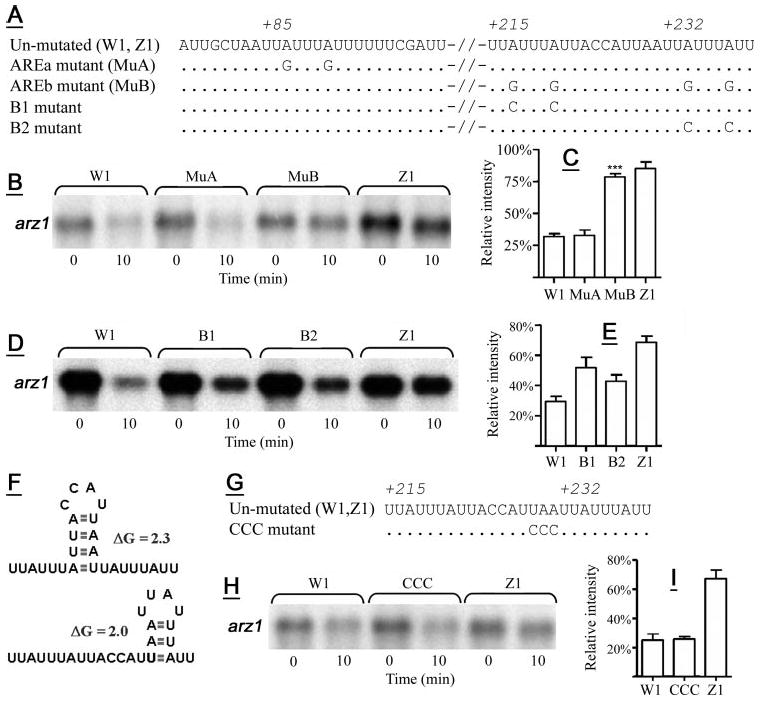
A, shown are the sequences of the arz1 ARE mutant constructs used for the experiments shown in B–E, with abbreviations for strains expressing these constructs in parentheses. Dots indicate identity with the un-mutated sequence, whereas mutated bases are specified. Numbers represent the base number in the arz1 3′-UTR, with 1 corresponding to the base immediately after the stop codon (base 2,326,651 in NCBI accession number NC_003421.2). In B, D, and H: W1 and Z1 are described in Fig. 1, whereas MuA, MuB, B1, B2, and CCC symbolize the wild-type strain transformed with the respective mutant constructs, which are named according to their specific mutations (A and F). B, D, and H, shown are Northern blots demonstrating arz1 mRNA levels in the respective transformant strains at 0 and 10 min after thiamine addition. C, shown are mean mRNA levels (±S.D.) of W1, MuA, MuB, or Z1 transformant strains from four separate experiments 10 min after the addition of thiamine. ***, p < 0.0001, when comparing MuB mRNA levels to those of W1. E, shown are the effects of the mutations introduced for the B1 and B2 transformant strains on arz1 mRNA decay at 10 min after thiamine repression, with means (±S.D.) from four separate experiments presented. Multiple mean comparisons of W1, B1, B2, and Z1 (E) using the Bonferroni multiple comparison test showed significant differences between all means. F, two potential stem-loop structures within AREb are shown, along with predicted initial ΔG values. G, shown are the mutations introduced in AREb predicted to prevent the formation of both of these possible structures. H, shown is the effect of the CCC mutation on zfs1-promoted mRNA decay observed at 10 min after thiamine repression, and in I are shown the mean values (±S.D.) of three independent identical experiments. In C, E, and I: arz1 mRNA levels at 10 min are presented after normalization by actin mRNA and calculated as a percentage of arz1 mRNA levels at 0 min for each strain, which was set to 100%.
Cladogram Construction
TZF domains from select proteins were aligned based on blastp (31, 32) results, and a representative cladogram was constructed using the neighbor-joining method and the p-distance substitution model with MEGA 3.1 (33). All TZF domains surveyed in this analysis were the same length, with the exception of the Cryptococcus neoformans TZF, which is three amino acids longer in the normally 18-amino acid spacer between zinc fingers and thus served as a natural out-group for comparison.
Homology Modeling
A model of the TZF domain segment of zfs1 (amino acids 327–390, NCBI accession number CAB75997.1) was obtained using Swiss-Model (34–36) by submitting the sequence through the First Approach mode. The resultant PDB file was examined, and the known structure of the ZFP36L2 TZF domain complexed with the ARE (13) was fit onto the zfs1 model using DeepView v3.7 (34), resulting in a combined file. A layer of this file was saved that included the zfs1 TZF domain and the ARE nonamer. Distances between core atoms present in specific functional groups were measured using DeepView v3.7, and POV Ray v3.6 was used for renderings.
Results
Transcripts Elevated in zfs1-deficient S. pombe
mRNAs obtained from S. pombe wild-type and zfs1-deficient strains were compared directly using the Yeast 2.0 array (Affymetrix, Santa Clara, CA). Results were deposited in the NCBI GEO (37, 38) (www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO series accession number GSE8122. 123 transcripts were elevated in the zfs1-deficient strain at mean levels ≥1.5- fold greater than in the wild-type strain; in all cases, the mean levels were significantly different as determined by Rosetta Resolver (22); p values are presented in Table 1. These transcripts were then examined for AREs of the TTP binding type present in the hypothetical 3′-UTR comprising 1 kb downstream of the stop codon. From the 123 significantly elevated transcripts, 12 contained two or more UAUUUAU sequences in this region (Table 1). From this analysis we chose to examine in more detail the most elevated candidate transcript containing likely binding sites, encoded at SPCC1494.03 in the S. pombe genome.
Elevation of arz1 mRNA Levels in the Absence of zfs1
Northern analysis of total RNA isolated in parallel from wild-type and zfs1-deficient strains (four separate cultures of each) revealed that the mRNA encoded by the SPCC1494.03 locus was elevated by 3.6-fold in the zfs1-deficient strain (Fig. 1, A and B), comparable to the microarray results (4.3 fold) (Table 1). We have named the protein encoded by SPCC1494.03 arz1 (signifying armadillo-repeat containing zfs1 target 1), based on the presence of armadillo-like (ARM) repeats in the arz1-predicted protein sequence (NCBI accession number CAA19301.1), and on apparent arz1 mRNA targeting by zfs1 (see below). arz1 has two different types of ARM repeat. The first repeats are found in three areas that encompass amino acids 7–177, 205–317, and 401–486, and the second is found at amino acids 346–428 (SPCC1494.03). The ARM repeats contribute to the high percentage of leucine (12%) in the arz1 protein sequence and result in limited amino acid sequence identity to ARM repeat-containing proteins such as human β-catenin (39, 40). Blast (31, 32) searches showed that the entire length of the deduced arz1 sequence aligns with several vertebrate Rap1-like sequences (at 20% identity) and revealed a stretch of 151 amino acids in arz1 with 25% identity to several ARM repeat-containing proteins in Drosophila, including Vimar (41). The function of S. pombe arz1 has not been explored, and it does not appear to have an S. cerevisiae orthologue.
Figure 1. Effect of zfs1 deficiency on arz1 mRNA stability.
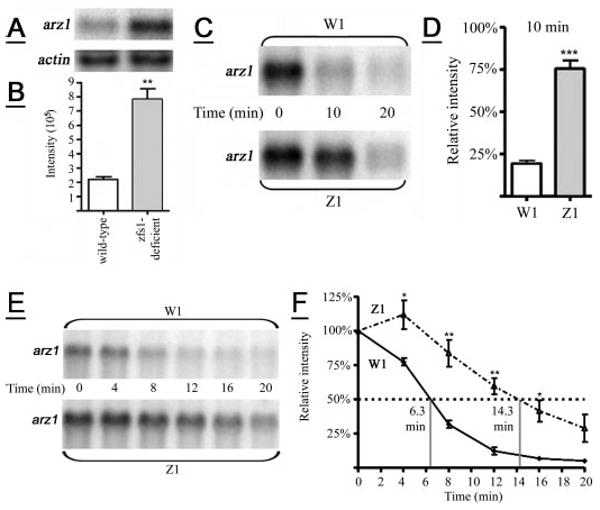
A, shown is a representative Northern blot illustrating the endogenous levels of arz1 mRNA from wild-type and zfs1-deficient S. pombe strains. The same blot was probed with an actin control for gel loading. B, shown is an analysis of mean phosphorimaging values (±S.D.) for arz1 mRNA levels (normalized for actin levels) from four separate experiments identical to those shown in A. **, p = 0.0012. C, shown are arz1 mRNA levels at 10 min after expression from the nmt/arz1 construct and transcription repression with thiamine. W1 and Z1 symbolize wild-type and zfs1-deficient strains, respectively, transformed with the nmt/arz1 construct. D, shown are the mean values (±S.D.) from five independent time courses of arz1 mRNA levels at 10 min, identical to that shown in C. ***, p < 0.0001. E, shown are arz1 mRNA levels after expression from the nmt/arz1 construct and transcription repression with thiamine. F, arz1 mRNA levels at 4, 8, 12, 16, and 20 min were calculated as a percentage of time 0 min (100%) after normalization to actin levels. Shown are the mean values (±S.D.) from four independent time courses of arz1 mRNA levels, identical to that shown in E.F, the solid line represents data from the W1 transformant strain, and the broken line represents data from the Z1 transformant strain. *, p < 0.05; **, p < 0.01. The half-life of the arz1 mRNA in each strain is noted.
Effect of zfs1 Deficiency on arz1 Transcript Decay
We measured arz1 mRNA stability in the presence or absence of zfs1, using the nmt (no message with thiamine) repressible expression system (23). Wild-type and zfs1-deficient strains transformed with the nmt/arz1 expression construct (named W1 and Z1, respectively) were examined for relative arz1 mRNA abundance after thiamine addition (repression), which was determined by normalizing arz1 mRNA levels to actin and calculating the percentage of arz1 mRNA present at each time point after repression. The first screen focused on evaluating arz1 mRNA levels at 10 or 20 min relative to 0 min (0 min equals 100% relative intensity). Ten minutes after the addition of thiamine, the relative abundance of the arz1 transcript in the Z1 strain was significantly greater than in the W1 strain (Fig. 1, C and D). By 20 min most of the arz1 transcript was degraded in both strains (Fig. 1, C and E). Data from five independent replicates of this experiment, in which mRNA levels from W1 and Z1 transformants at 10 min were compared directly, are summarized in Fig. 1D. The half-life of the arz1 mRNA in the W1 and Z1 strains was then evaluated by sampling earlier time points (at 4 and 8 min) continuing on to 20 min in 4-min intervals (Fig. 1, E and F). Data from four independent replicates of this experiment, in which mRNA levels from W1 and Z1 transformants were compared directly, are summarized in Fig. 1F. The arz1 mRNA half-life was extended from ∼6.3 min in the W1 strain to 14.3 min in the Z1 strain (Fig. 1F).
ARE Dependence of zfs1-mediated arz1 mRNA Decay
Three UUAUUUAUU nonamers are present in the predicted arz1 3′-UTR, including one at 85 bases 3′ of the stop codon (AREa), and two others at 215 and 232 bases 3′ of the stop codon (named AREb, collectively) (Fig. 2A). These ideal TTP binding sites were mutated to UUGUUUGUU in each ARE (Fig. 2A). We compared transcript levels at 10 min after thiamine addition between the following strains: W1 (described above), MuA (wild-type S. pombe expressing arz1 mRNA with mutations A87G and A91G) or MuB (wild-type S. pombe expressing arz1 mRNA with four mutations A217G, A221G, A224G, and A238G), and Z1 (described above) (Fig. 2A). For each comparison, mean arz1 mRNA levels at 10 min are shown as normalized to actin control mRNA levels and as a calculated percentage of the respective 0-min mRNA level, to indicate relative arz1 mRNA abundance at 10 min. Mean arz1 mRNA levels observed in the MuA mutant were identical to those observed for W1 (Fig. 2B), indicating that mutations at AREa did not confer a protective effect on the arz1 mRNA. The MuB mutant arz1 mRNA levels were comparable to those observed for Z1 and significantly greater than those observed for W1 (Fig. 2C), indicating that the mutations at AREb protected against zfs1-dependent decay. This strongly supports a role for AREb, but not AREa, in zfs1-mediated arz1 transcript destabilization.
Mutations in each of the core UUAUUUAUU sequences within AREb separately (B1 and B2 mutants (Fig. 2, D and E) resulted in only partial protection from zfs1-mediated decay (Fig. 2D). The B1 mutant, expressing arz1 mRNA containing both the A217C and A221C mutations, appeared to confer greater protection from decay than did the MuB2 mutant (Fig. 2, D and E). A one-way analysis of variance of mean arz1 mRNA levels from W1, B1, B2, and Z1 strains indicated significant variance across multiple means (p < 0.0001). Using the Bonferroni multiple comparison test (Fig. 2E), we showed that B1 and B2 mutant arz1 mRNA levels were different than those seen in both the W1 and Z1 transformant strains (p < 0.001, with the exception of W1 versus B2, which was p < 0.01, and B1 versus B2 was p < 0.05).
Two separate stem-loop structures were predicted in the AREb sequence (Fig. 2F): one (initial ΔG = 2.3 kcal/mol) that could juxtapose the two nonamers into an overlapping binding site, and another within the B2 nonamer (initial ΔG = 2.0 kcal/mol). To examine the role of these possible structures in zfs1-mediated mRNA decay, we mutated three residues (U229–A231) predicted to participate in both structures (Fig. 2, F and G), resulting in a change from 5′-UAA-3′ to 5′-CCC-3′ in the expressed mRNA (Fig. 2F), thus preventing the formation of both of the predicted stem-loop structures. However, mutating these residues had no effect on zfs1-dependent arz1 transcript decay (Fig. 2, H and I).
zfs1 Binding to ARE Probes
The ability of recombinant zfs1 to bind directly to ARE-based RNA probes was assessed using RNA gel shift assays. MBP/zfs1, two separate zinc finger mutant forms of MBP/zfs1 (C370G and H351I), and MBP alone were expressed in and purified from E. coli. Fig. 3A shows that the recombinant proteins were quite pure based on SDS-PAGE.
Figure 3. Gel shift of recombinant zfs1 with ARE-containing RNA oligonucleotides.
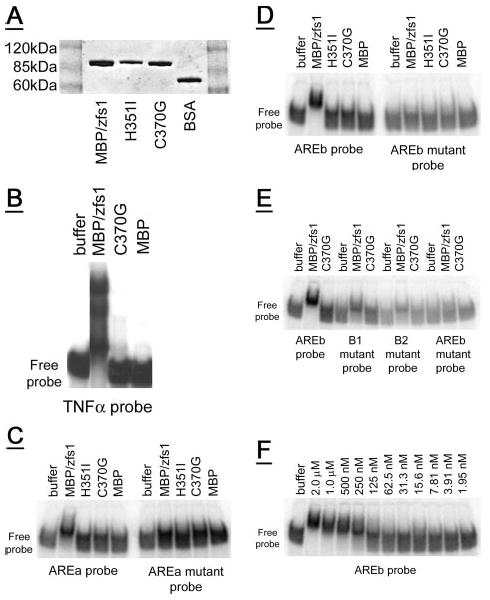
A, a Coomassie Blue-stained SDS-PAGE gel showing recombinant MBP/zfs1 (0.6 μg) compared with recombinant MBP/zfs1 zinc finger mutants (C370G, 0.5 μg, and H351I, 0.4 μg), and albumin (bovine serum albumin, 0.5 μg). Molecular weight marker positions are indicated. B, MBP/zfs1 completely shifted the tumor necrosis factor-α-based probe into the gel, whereas neither the C370G amino acid mutant of MBP/zfs1, nor MBP alone, caused the same gel shift. C, a partial shift is observed for an arz1 AREa-based probe with MBP/zfs1, but not with either zinc finger mutant MBP/zfs1 protein or MBP alone. Mutations in the binding site of the AREa probe eliminated the partial shift observed with MBP/zfs1. D, an AREb-based probe is completely shifted into the gel by MBP/zfs1, but not with either zinc finger mutant MBP/zfs1 protein or MBP alone. Mutations in the two binding sites of the AREb probe eliminated the shift observed with MBP/zfs1. E, mutations in either AREb binding site (B1 or B2) resulted in a partial shift. 50 pmol of each protein was assayed. F, a serial dilution of 50 pmol of MBP/zfs1 was used in the gel shift assay with the AREb probe, resulting in the range of concentrations shown. Half-maximal binding of the AREb probe (100 fmol total, 4 nm final concentration) was observed with ∼3.125–6.25 pmol of MBP/zfs1 (125–250 nm final concentration). For each gel (B–F) the position of the free probe at the bottom of each gel is indicated.
When equal amounts of MBP/zfs1, C370G and MBP alone were allowed to bind the tumor necrosis factor-α probe, the probe was completely shifted by the recombinant MBP/zfs1 protein (Fig. 3B), whereas neither the MBP/zfs1 zinc finger mutant, C370G, nor the MBP protein alone caused analogous shifting of the probes (Fig. 3B). A small fraction of probe was shifted into the gel by both the C370G mutant and the MBP protein alone, indicating that some zinc finger independent or nonspecific binding to these probes can occur in these conditions.
Several probes based on arz1 AREa and AREb were examined by gel shift in the presence of MBP/zfs1 recombinant proteins and MBP alone. A probe based on the AREa sequence was partially shifted by 50 pmol of MBP/zfs1 (2 μm) (Fig. 3C), but not by either zinc finger mutant form or by MBP alone (Fig. 3C). A mutant probe with alterations in the predicted binding site at AREa (Table 4) was not shifted by MBP/zfs1 (Fig. 3C). An AREb-based probe was completely shifted by 50 pmol of MBP/zfs1 (2 μm) (Fig. 3D), but not by equal amounts of either zinc finger mutant or by MBP alone (Fig. 3D). A mutant probe with alterations in both predicted binding sites at AREb (Table 4) was not shifted by MBP/zfs1 (Fig. 3D). AREb mutant probes, based on the mutations examined in Fig. 2 (D and E) using the nmt/arz1 expression system, were also tested (Table 4). Fig. 3E shows a complete shift of the AREb probe by MBP/zfs1, with partial shifts observed for the B1 and B2 mutant probes.
A serial dilution of the MBP/zfs1 protein starting from 2 μm was applied to the AREb probe to examine the affinity of this interaction. Half-maximal binding appeared to occur at 125–250 nm, which was thus in excess over the 4 nm of probe used in the assays. It is possible that either the assay conditions or the recombinant production system yielded a fraction of protein that would not bind probe.
zfs1 mRNA and Iron Deprivation
Following methods described previously for the study of S. cerevisiae CTH2 (19), the S. pombe wild-type strain was grown in iron-deficient conditions in parallel with normal conditions. Growth rates were similar under the two different conditions. Previously, zfs1 mRNA was not observed to be affected by iron addition or depletion (20). Our results confirmed those findings (20) (data not shown). This result contrasts sharply with the dramatic increase of CTH2 mRNA observed in S. cerevisiae (19) after iron depletion.
Comparison of the zfs1 TZF Domain to Other Eukaryotic TZF Domains
Analysis of selected eukaryotic sequences revealed that the S. pombe zfs1 TZF domain exhibited more amino acid sequence identity to TZF domain sequences from metazoans than to those from other yeasts (Fig. 4), whereas the two S. cerevisiae TZF domain sequences (from CTH1 and CTH2) (18) cluster with TZF domain sequences from two Candida species, C. albicans and C. glabrata (Fig. 4B).
Figure 4. Comparison of the zfs1 TZF domain to other TTP family members.
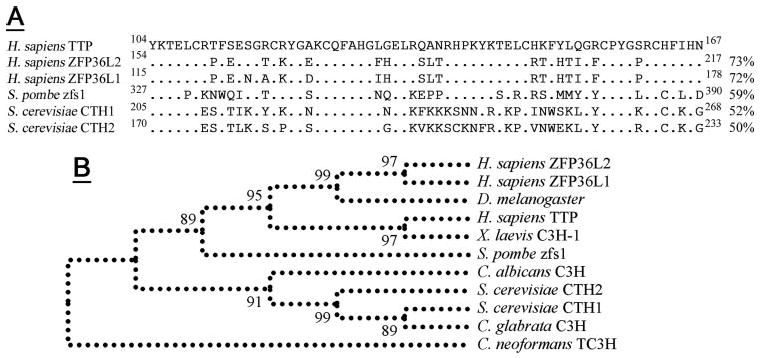
In A the zfs1 TZF is aligned with the three human TTP family members and the two family members expressed in S. cerevisiae. Percent amino acid identity with the human TTP TZF domain is shown (right) and identical residues to the human TTP sequence are indicated (dots). Conserved residues are underlined (top), with key Cys and His residues indicated (asterisks). The cladogram presented (B) shows how the zfs1 TZF domain forms a natural root to the cluster of metazoan TZFs. Other yeast TZF domains form a separate cluster, with a C. neoformans TZF domain forming a natural out-group. Bootstrap values are from 500 replicates. NCBI refseq accession numbers are as follows: Homo sapiens TTP, NP_03398.1; H. sapiens ZFP36L2, NP_008818.3; H. sapiens ZFP36L1, NP_004917.2; Xenopus laevis C3H-1, NP_001081885.1; Drosophila melanogaster Tis11, NP_511141.2; S. pombe zfs1, CAB75997.1; S. cerevisiae CTH1, P47976; S. cerevisiae CTH2, P47977; C. albicans, XP_717137.1; C. glabrata, XP_445742.1; and C. neoformans, EAL17658.1.
Conservation of Key TZF Domain Motif Characteristics in zfs1
A model of the zfs1 TZF domain made using Swiss-Model (36) revealed that conserved residues between TTP and zfs1 (Fig. 4) are found in the general ARE interaction areas (Fig. 5A). Stacking interactions are predicted between highly conserved aromatic side chains and RNA bases (Fig. 5A). Main polypeptide chain interactions with RNA bases are predicted to be involved in the TZF domain-ARE interaction (Fig. 5B), as observed for ZFP36L2 (13). The polypeptide chain of the N-terminal zinc finger at zfs1 amino acids Glu-330 and Pro-331 may hydrogen bond with the adenosine residue (A7), an interaction that is in close proximity to the zinc binding core. The side chain of a conserved Arg residue in the C-terminal finger (Fig. 5A, Arg-383) extends into the N-terminal zinc-finger (Fig. 5, A and B). This interaction may be important to the overall configuration of the TZF domain (Fig. 5B).
Discussion
The most important findings of this study are that zfs1 is an ARE-specific mRNA-binding protein that exhibits binding site selectivity and can promote mRNA decay, and that the arz1 transcript is the first target identified for zfs1. A function has not been determined for the arz1 protein, but the regulation of arz1 transcript levels by zfs1 may eventually provide insights into the role of zfs1 in cell division and mating. The system we describe for assessing zfs1-mediated mRNA decay holds promise for the evaluation of the mechanisms of action of all the TTP family proteins. S. pombe expresses only a single TTP family member, whereas humans express three TTP family members. zfs1 can be studied at native expression levels in vivo, whereas most studies of TTP and its relatives have relied on transfection and overexpression studies in cultured cells.
Our findings provide evidence of binding site selectivity in a native mRNA targeted by a TTP family member. Specifically, non-binding mutations within the arz1 5′-most binding site present in AREa had no effect on zfs1-dependent decay, despite the fact that its sequence corresponds to that of an optimal TTP family member binding site (11, 13). In contrast, non-binding mutations within the two 3′-most arz1 binding sites (AREb) essentially eliminated zfs1-dependent decay. Further evidence of binding site selectivity was observed when mutations within each of the binding sites present in AREb were introduced, each resulting in partial elimination of zfs1-dependent decay. Based on what is known from the NMR structure of the ZFP36L2 TZF domain bound to the optimal ARE nonamer, we can postulate that several interactions occur between amines in the adenine bases and both main chain and side chain functional groups in the zfs1 TZF domain. These interactions probably form the basis for binding affinity that results in biological function (i.e. mRNA decay).
Binding site selectivity may be explained by the propensity for tandem nonamers to contribute in an interactive fashion to the targeting of this region by zfs1 in a way that facilitates mRNA decay, although a previous study showed that a single nonamer binding site can confer TTP sensitivity to a previously insensitive transcript (10). The gel shift assays showed that an RNA probe based on the AREa sequence could be partially shifted by recombinant zfs1, but only at concentrations ∼10-fold higher than that needed to generate a similar shift with the AREb based probe. It is possible that the binding site present at AREa was not accessible to zfs1 due to mRNA secondary structure, or the presence of other binding proteins. In contrast, AREb was clearly accessible, and one of the nonamers within AREb was favored for biological activity. Our results reflect the possible cooperative nature of zfs1 function, because each nonamer binding site within AREb appeared to contribute to zfs1 sensitivity in an additive fashion.
The AREb of arz1 spans a section of this transcript that has the potential to form at least two stem-loop structures that could potentially affect the exposed single-stranded portions of the two nonamers. Our results show that the formation of either of these structures, if formed in vivo, should not influence the activity of zfs1.
arz1, the newly identified zfs1 target transcript, encodes a protein that exhibits a low level of amino acid sequence similarity to metazoan signal transduction proteins, including members of the armadillo/β-catenin family. The arz1 protein appears to be distributed in both the cytoplasm and the nucleus (42), which supports this resemblance to the armadillo/β-catenin proteins (43, 44). The known roles of zfs1 in regulating reproduction and cell division presumably depend on its ability to regulate gene expression by controlling mRNA decay; such roles are probably executed by proteins encoded by zfs1 target mRNAs such as arz1. An indirect role of zfs1 in such processes would explain the lack of a direct association between zfs1 and proteins known to be involved in either pheromone signal transduction or septum formation, as noted previously (16, 17).
zfs1 is very different from CTH2, a TZF domain protein in S. cerevisiae that is induced by iron depletion, resulting in down-regulation of mRNAs involved in iron metabolism (19). We found that zfs1 mRNA was not induced by iron depletion in S. pombe, corroborating a previous report stating that zfs1 mRNA levels are not influenced by iron addition or depletion (20). Moreover, zfs1 deletion had no effect on transcripts encoding proteins involved in iron metabolism or on their regulation by iron availability (20). The other TZF protein in S. cerevisiae, CTH1, has not yet been characterized functionally, but resembles zfs1 no more than CTH2, with both proteins exhibiting 52% identity to zfs1 in the TZF domain. Interestingly, the TZF domain of zfs1 resembles that of human TTP (59% identity) more closely than do the S. cerevisiae CTH1 or CTH2 TZF domains (52 and 50%, respectively), making it a better root to metazoan TZF domain sequences than either of the S. cerevisiae proteins in phylogenetic trees. These factors emphasize the potential that S. pombe zfs1 presents as a model for the study of TTP family proteins.
The S. pombe nmt expression system provides a useful alternative to mammalian transient transfection studies for the examination of TZF domain-ARE interactions. The limitations of mammalian transfection systems include difficulties in controlling the timing and extent of the co-expression and the artificially high levels of expression of the TTP family members. The nmt expression system appears to be well suited to such studies because of the rapidly repressible nature of the promoter, permitting direct assessment of mRNA decay, in the context of the single TTP family member, zfs1, expressed at endogenous levels. Furthermore, the genetic tractability of yeasts permits the development of important strains, such as the zfs1-deficient strain, which may facilitate the evaluation of the importance of cofactors and modifying proteins in the regulation of accelerated target transcript decay.
The function of zfs1 in S. pombe to regulate the pheromone response and cell division should provide new insights into the relevance of mRNA decay in the control of these processes. Future investigations will focus on the biochemical role of zfs1 targets in these physiological events.
Acknowledgments
We thank Viesturs Simanis for the S. pombe strains, Will Gersch for assistance, Joe Krahn for help with modeling, Debbie Stumpo for help with Northern blots, the MBP/zfs1 expression construct and helpful discussions, and Wi Lai for help with the RNA gel shift assays and helpful discussions. We thank members of the NIEHS microarray facility for their help on this project, and Daniel Menendez and Yong-Sik Kim for critical review of this manuscript.
Footnotes
This work was supported by the intramural program of NIEHS, National Institutes of Health.
The abbreviations used are: TTP, tristetraprolin; TZF, CCCH tandem zinc finger; ARE, AU-rich element; nmt, no message with thiamine; arz1, armadillo-repeat containing zfs1 target 1; ARM, armadillo-repeat; GEO, NCBI Gene Expression Omnibus; EMM, Edinburgh minimal medium; MBP, maltose-binding protein.
References
- 1.Lai WS, Parker JS, Grissom SF, Stumpo DJ, Blackshear PJ. Mol Cell Biol. 2006;26:9196–9208. doi: 10.1128/MCB.00945-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackshear PJ. Biochem Soc Trans. 2002;30:945–952. doi: 10.1042/bst0300945. [DOI] [PubMed] [Google Scholar]
- 3.Lai WS, Carballo E, Strum JR, Kennington EA, Phillips RS, Blackshear PJ. Mol Cell Biol. 1999;19:4311–4323. doi: 10.1128/mcb.19.6.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carballo E, Lai WS, Blackshear PJ. Science. 1998;281:1001–1005. doi: 10.1126/science.281.5379.1001. [DOI] [PubMed] [Google Scholar]
- 5.Ogilvie RL, Abelson M, Hau HH, Vlasova I, Blackshear PJ, Bohjanen PR. J Immunol. 2005;174:953–961. doi: 10.4049/jimmunol.174.2.953. [DOI] [PubMed] [Google Scholar]
- 6.Carballo E, Lai WS, Blackshear PJ. Blood. 2000;95:1891–1899. [PubMed] [Google Scholar]
- 7.Barnard RC, Pascall JC, Brown KD, McKay IA, Williams NS, Bustin SA. Nucleic Acids Res. 1993;21:3580. doi: 10.1093/nar/21.15.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nie XF, Maclean KN, Kumar V, McKay IA, Bustin SA. Gene (Amst) 1995;152:285–286. doi: 10.1016/0378-1119(94)00696-p. [DOI] [PubMed] [Google Scholar]
- 9.Brewer BY, Ballin JD, Fialcowitz-White EJ, Blackshear PJ, Wilson GM. Biochemistry. 2006;45:13807–13817. doi: 10.1021/bi061320j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai WS, Carrick DM, Blackshear PJ. J Biol Chem. 2005;280:34365–34377. doi: 10.1074/jbc.M506757200. [DOI] [PubMed] [Google Scholar]
- 11.Brewer BY, Malicka J, Blackshear PJ, Wilson GM. J Biol Chem. 2004;279:27870–27877. doi: 10.1074/jbc.M402551200. [DOI] [PubMed] [Google Scholar]
- 12.Blackshear PJ, Lai WS, Kennington EA, Brewer G, Wilson GM, Guan X, Zhou P. J Biol Chem. 2003;278:19947–19955. doi: 10.1074/jbc.M301290200. [DOI] [PubMed] [Google Scholar]
- 13.Hudson BP, Martinez-Yamout MA, Dyson HJ, Wright PE. Nat Struct Mol Biol. 2004;11:257–264. doi: 10.1038/nsmb738. [DOI] [PubMed] [Google Scholar]
- 14.Lai WS, Kennington EA, Blackshear PJ. J Biol Chem. 2002;277:9606–9613. doi: 10.1074/jbc.M110395200. [DOI] [PubMed] [Google Scholar]
- 15.Lai WS, Kennington EA, Blackshear PJ. Mol Cell Biol. 2003;23:3798–3812. doi: 10.1128/MCB.23.11.3798-3812.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanoh J, Sugimoto A, Yamamoto M. Mol Biol Cell. 1995;6:1185–1195. doi: 10.1091/mbc.6.9.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beltraminelli N, Murone M, Simanis V. J Cell Sci. 1999;112:3103–3114. doi: 10.1242/jcs.112.18.3103. [DOI] [PubMed] [Google Scholar]
- 18.Thompson MJ, Lai WS, Taylor GA, Blackshear PJ. Gene (Amst) 1996;174:225–233. doi: 10.1016/0378-1119(96)00084-4. [DOI] [PubMed] [Google Scholar]
- 19.Puig S, Askeland E, Thiele DJ. Cell. 2005;120:99–110. doi: 10.1016/j.cell.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 20.Mercier A, Pelletier B, Labbe S. Eukaryot Cell. 2006;5:1866–1881. doi: 10.1128/EC.00199-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Labbe S, Pelletier B, Mercier A. Biometals. 2007;20:523–537. doi: 10.1007/s10534-006-9056-5. [DOI] [PubMed] [Google Scholar]
- 22.Weng L, Dai H, Zhan Y, He Y, Stepaniants SB, Bassett DE. Bioinformatics. 2006;22:1111–1121. doi: 10.1093/bioinformatics/btl045. [DOI] [PubMed] [Google Scholar]
- 23.Maundrell K. Gene (Amst) 1993;123:127–130. doi: 10.1016/0378-1119(93)90551-d. [DOI] [PubMed] [Google Scholar]
- 24.Basi G, Schmid E, Maundrell K. Gene (Amst) 1993;123:131–136. doi: 10.1016/0378-1119(93)90552-e. [DOI] [PubMed] [Google Scholar]
- 25.Sanger F, Nicklen S, Coulson AR. Proc Natl Acad Sci U S A. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beach D, Piper M, Nurse P. Mol Gen Genet. 1982;187:326–329. doi: 10.1007/BF00331138. [DOI] [PubMed] [Google Scholar]
- 27.Okazaki K, Okazaki N, Kume K, Jinno S, Tanaka K, Okayama H. Nucleic Acids Res. 1990;18:6485–6489. doi: 10.1093/nar/18.22.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch EFM, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 1989. [Google Scholar]
- 29.Kunkel TA. Proc Natl Acad Sci U S A. 1985;82:488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuker M. Nucleic Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 32.McGinnis S, Madden TL. Nucleic Acids Res. 2004;32:W20–W25. doi: 10.1093/nar/gkh435. web server issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar S, Tamura K, Nei M. Brief Bioinform. 2004;5:150–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- 34.Guex N, Peitsch MC. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 35.Schwede T, Diemand A, Guex N, Peitsch MC. Res Microbiol. 2000;151:107–112. doi: 10.1016/s0923-2508(00)00121-2. [DOI] [PubMed] [Google Scholar]
- 36.Schwede T, Kopp J, Guex N, Peitsch MC. Nucleic Acids Res. 2003;31:3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barrett T, Troup DB, Wilhite SE, Ledoux P, Rudnev D, Evangelista C, Kim IF, Soboleva A, Tomashevsky M, Edgar R. Nucleic Acids Res. 2007;35:D760–765. doi: 10.1093/nar/gkl887. database issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edgar R, Domrachev M, Lash AE. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peifer M, Berg S, Reynolds AB. Cell. 1994;76:789–791. doi: 10.1016/0092-8674(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 40.Gumbiner BM. Curr Opin Cell Biol. 1995;7:634–640. doi: 10.1016/0955-0674(95)80104-9. [DOI] [PubMed] [Google Scholar]
- 41.Lo PC, Frasch M. Mech Dev. 1998;72:65–75. doi: 10.1016/s0925-4773(98)00016-1. [DOI] [PubMed] [Google Scholar]
- 42.Matsuyama A, Arai R, Yashiroda Y, Shirai A, Kamata A, Sekido S, Kobayashi Y, Hashimoto A, Hamamoto M, Hiraoka Y, Horinouchi S, Yoshida M. Nat Biotechnol. 2006;24:841–847. doi: 10.1038/nbt1222. [DOI] [PubMed] [Google Scholar]
- 43.Stadeli R, Hoffmans R, Basler K. Curr Biol. 2006;16:R378–R385. doi: 10.1016/j.cub.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 44.Coates JC. Trends Cell Biol. 2003;13:463–471. doi: 10.1016/s0962-8924(03)00167-3. [DOI] [PubMed] [Google Scholar]


