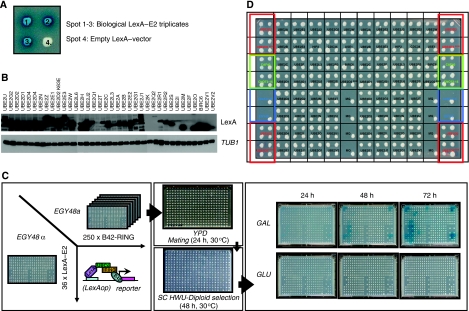
Figure 1.
Outline array based, mating yeast two-hybrid matrix screen. (A) Organization of LexA–E2 fusions as three independent bacterial triplicate clones and an empty LexA vector. (B) LexA–E2 protein expression levels. Total protein lysates of yeast cells transformed with LexA–E2 fusions were resolved on SDS–PAGE, transferred to membranes and probed with either LexA antibody or yeast tubulin (TUB1). (C) Overview of experimental yeast two-hybrid screening procedure. EGY48α cells were transformed with 36 LexA–E2 fusions and EGY48a cells were transformed with 250 B42–RING-fusions. Screening for pair-wise E2–E3 interactions was carried out by standardized array-based mating between EGY48α and EGY48a cells on YPD for 24 h at 30°C, followed by diploid selection on SC HWU− for 48 h at 30°C. Interactions were visualized by transferring diploids on SC HWU− X-Gal (colorimetric selection) or on SC HWUL− (auxotrophic selection) under both B42-inducing (galactose as main carbon source) and repressive (glucose) conditions. Digital images of the interactions were taken at 3, 24-h interval time points. (D) Overview of standardized LexA–E2 array. Each spot represents EGY48α cells transformed with the indicated LexA–E2 fusion construct. Spots with a red square contained LexA–UBE2D2 and LexA–UBE2D2 K63E constructs and were mated throughout the entire procedure with EGY48a cells transformed with B42–CNOT4 N63 as positive and negative interaction controls, respectively. Spots with a green square were transformed with LexA–Caf40 and LexA–CNOT2-15 and served as auto-activating controls. Blue squared spots represent either EGY48a cells transformed with LexA-empty vector and an untransformed EGY48α strain, as mating/growing controls.