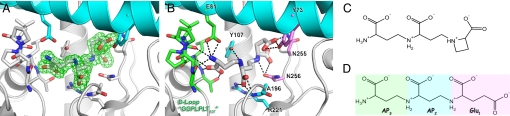
Fig. 2.
Identification and environment of tNA. (A) Electron density map (Fo-Fc contoured at 2.5σ) around the tNA molecule. The residues involved in the binding of tNA are shown in stick form and colored according to the domain to which they belong (cyan, N-terminal domain; white, C-terminal domain). (B) As in A, hydrogen bonds are shown as dotted lines. Residues stabilizing the AP3, AP2, and Glu1 moieties are colored in green, blue, and magenta, respectively. Note the seven hydrogen bonds involving the terminal aminopropyl moiety (AP3). (C–D) Chemical structures of NA (C) and tNA (D).