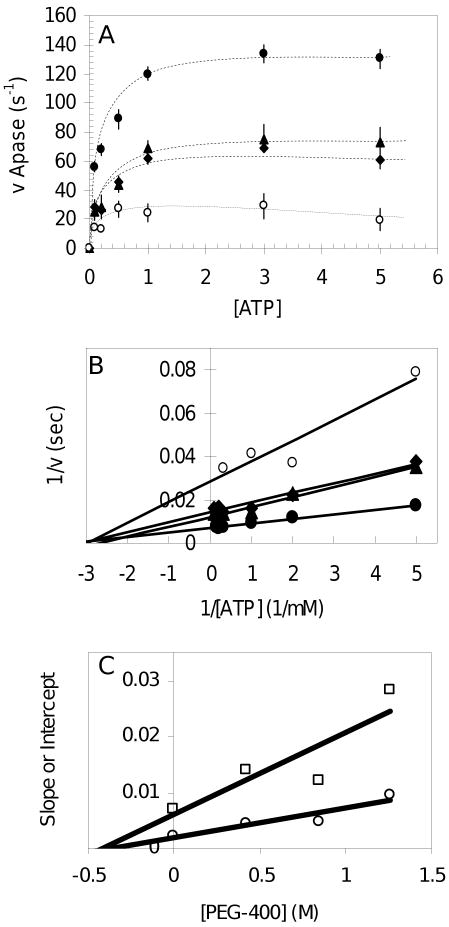
Fig. 1.
Noncompetitive inhibition of F1-ATPase activity by PEG-400. (A) Effect of PEG-400 on the bulk ATPase activity of F1 without nanorods. The linear initial rate of F1 ATPase activity was determined in triplicate with phenol red as indicated in Methods using 10 μg of F1 in the presence of (●) 0% PEG-400, (▲) 15% PEG-400, (◆) 30% PEG-400, (○) 45% PEG-400 (v/v). (B) Double reciprocal plot of the data from (A). (C) Plot of the the slopes (□) and y-intercepts (○) of each trend line from Figure 1B versus PEG-400 concentration. The absolute value of the x-intercepts determined by linear regression are defined as the inhibition constants Kis (slopes) and Kii (intercepts).