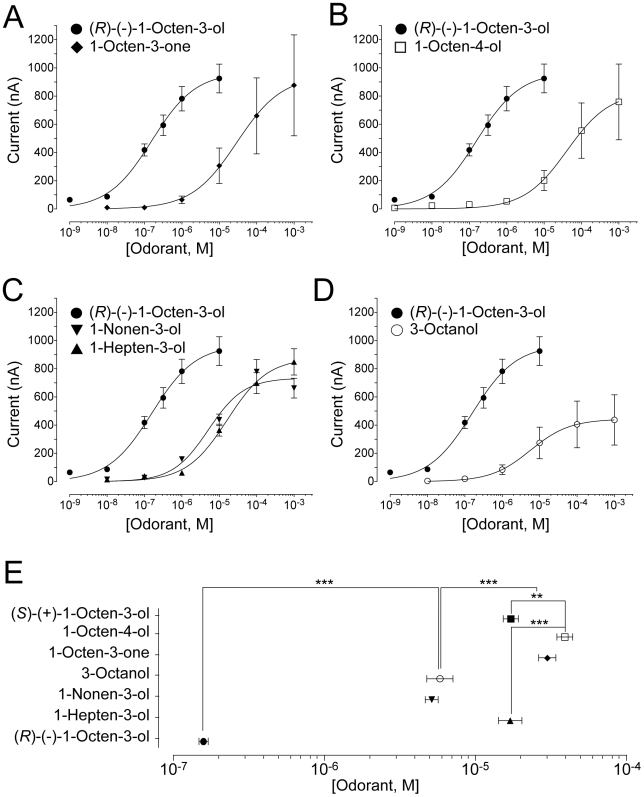
Figure 2. Strong preference of AaOR8 towards (R)-(—)-1-octen-3-ol.
The concentration-response plot for (R)-(—)-1-octen-3-ol was repeated in each panel for comparative purposes. (A) Importance of C3 as a chiral center. Concentration-response plots of AaOR8 to 1-octen-3-one (n = 6). (B) Shifting the chiral center from C3 to C4 reduces AaOR8 sensitivity. Concentration-response plots of AaOR8 to 1-octen-4-ol (n = 8). (C) Side chain length affects AaOR8 sensitivity. Concentration-response plots of AaOR8 to 1-nonen-3-ol and 1-hepten-3-ol (n = 8 to 9). (D) The double bond is critical for recognition by AaOR8. Concentration-response plots of AaOR8 to 3-octanol (n = 6). (E) EC50 ranking profile of AaOR8 for octenol related compounds. Asterisk, p<0.05; two asterisks, p<0.01 and three asterisks, p<0.001. Odorant concentrations were plotted on a logarithmic scale. Each point represents the mean and error bars indicate s.e.m.