Abstract
CD4+CD25+Foxp3+ natural regulatory T cells (T reg cells) maintain self-tolerance and suppress autoimmune diseases such as type 1 diabetes and inflammatory bowel disease (IBD). In addition to their effects on T cells, T reg cells are essential for maintaining normal numbers of dendritic cells (DCs): when T reg cells are depleted, there is a compensatory Flt3-dependent increase in DCs. However, little is known about how T reg cell homeostasis is maintained in vivo. We demonstrate the existence of a feedback regulatory loop between DCs and T reg cells. We find that loss of DCs leads to a loss of T reg cells, and that the remaining T reg cells exhibit decreased Foxp3 expression. The DC-dependent loss in T reg cells leads to an increase in the number of T cells producing inflammatory cytokines, such as interferon γ and interleukin 17. Conversely, increasing the number of DCs leads to increased T reg cell division and accumulation by a mechanism that requires major histocompatibility complex II expression on DCs. The increase in T reg cells induced by DC expansion is sufficient to prevent type 1 autoimmune diabetes and IBD, which suggests that interference with this feedback loop will create new opportunities for immune-based therapies.
CD4+CD25+Foxp3+ natural regulatory T cells (T reg cells) are essential for maintaining self-tolerance (Kim et al., 2007; Sakaguchi et al., 2008). The loss of these cells leads to a fatal autoimmune syndrome affecting multiple organs (Sakaguchi et al., 1995; Kronenberg and Rudensky, 2005). In addition, these cells interfere with the development of organ-specific autoimmune diseases, such as type 1 diabetes (Salomon et al., 2000; Tarbell et al., 2004; You et al., 2008) and inflammatory bowel disease (IBD), by silencing self-reactive Th1 and Th17 cells (Powrie et al., 1993; Izcue et al., 2006; Korn et al., 2007).
Several requirements for T reg cell homeostasis in vivo have been defined. For example, T reg cells are IL-2 dependent and maintained by constant homeostatic division in response to self-antigens (Fisson et al., 2003; von Boehmer, 2003; Setoguchi et al., 2005). In addition to self-antigen recognition, which is essential for their activation and function (Thornton and Shevach, 1998; Samy et al., 2005), T reg cell survival in the periphery also requires co-stimulation through the CD28 and B7 pathway (Salomon et al., 2000). Finally, actively dividing T reg cells appear to be more suppressive than those that are quiescent (Klein et al., 2003). However, it is not known whether antigen-presenting cells (and which ones, if any) are required for maintaining T reg cells in vivo in the steady state (Denning et al., 2007; Yamazaki et al., 2008).
DCs are specialized antigen-presenting cells that capture, process, and present antigens to T cells (Banchereau and Steinman, 1998). The outcome of the encounter between these two cell types depends on the activation status of the DC. In the steady state, antigen presentation by DCs leads to tolerance by T cell deletion, induction of anergy, or expansion of antigen-specific T reg cells (Brocker et al., 1997; Hawiger et al., 2001; Steinman and Nussenzweig, 2002; Hawiger et al., 2004; Kretschmer et al., 2005; Luckashenak et al., 2008; Yamazaki et al., 2008). In contrast, antigen presentation by DCs that are activated or matured by Toll-like receptor ligands, CD40 ligation, Fc receptor signaling, or inflammatory cytokines leads to protective T cell immunity (Steinman and Nussenzweig, 2002). Given the importance of DCs for immune activation, it might be expected that the loss of these cells would lead to the absence of immune responses. However, congenital DC deficiency leads instead to a complex generalized lympho- and myeloproliferative syndrome with some features of autoimmune disease, including increased numbers of granulocytes, inflammatory mediators, and possibly T reg cells (Birnberg et al., 2008; Ohnmacht et al., 2009). Additional clues that DCs are involved in T reg cell homeostasis are given in the recent report that Flt3 ligand (FL) can expand T reg cells (Swee et al., 2009), and that loss of T reg cells increases DC division by a FL-dependent mechanism (Liu et al., 2009). Whether these effects on DCs result in direct or indirect feedback changes in T reg cell homeostasis in vivo is not known (Birnberg et al., 2008). In this report, we examined whether DCs are required to maintain T reg cells in vivo and uncovered the existence of a feedback regulatory loop required to maintain physiological numbers of the two cell types in the steady state.
RESULTS AND DISCUSSION
Numbers of T reg cells correlate with DCs
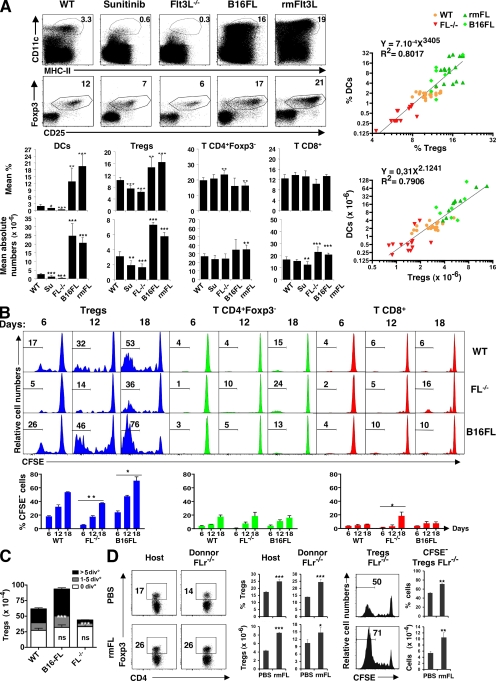
T reg cell depletion results in increased numbers of DCs by an FL-dependent mechanism (Liu et al., 2009). Conversely, antigens delivered to peripheral DCs in the steady state can induce antigen-specific T reg cell development and expansion, but the role of DCs in maintaining T reg cell homeostasis in vivo has not been determined. To ascertain whether DCs are required to maintain T reg cell homeostasis in vivo, we examined FL-deficient mice (FL−/− mice), which show a 10-fold reduction in the number of conventional DCs in the spleen and LN (Fig. 1 A; McKenna et al., 2000). We found a twofold reduction in the numbers of T reg cells (CD4+CD25+Foxp3+) in the spleen in the FL−/− mice. Similar effects were obtained by treating mice with sunitinib (O'Farrell et al., 2003), an Flt3 receptor (FLr) inhibitor (Fig. 1 A). Conversely, increasing the numbers of DCs by injecting mice with soluble FL (Maraskovsky et al., 1996; Swee et al., 2009) or B16 melanoma cells secreting FL was associated with an increase in the number of T reg cells in the spleen (Fig. 1 A). Plotting the number of DCs versus T reg cells in all such experiments revealed a highly significant and correlated relationship between the two cell types, both in the spleen and the LN (Fig. 1 A and Fig. S1). Other lymphoid populations, such as CD4+ and CD8+ T cells, were much less affected, and B cell depletion had no measurable effect on T reg cells (Fig. 1 A and Fig. S2). We conclude that the number of T reg cells is directly proportional to the number of DCs in vivo.
Figure 1.
Coordinate DC and T reg cell homeostasis. (A) Representative dot plots show the percentage of CD11chiMHCII+ cells (top) and Foxp3+CD25+ T reg cells (bottom) among CD3+CD4+ splenocytes. Numbers indicate percentages. C57BL/6 mice were untreated (WT), treated for 1 wk with sunitinib, injected with B16-FL tumor (B16FL), treated for 12 d with rmFL, or untreated FL−/− mice. Bar graphs show corresponding mean percentages and absolute numbers ± SD (n = 4–12 mice in five experiments). Graphs (right) show nonlinear regression analysis of data shown in histograms, plotting the percentage or absolute numbers of DCs (y axis) versus T reg cells (x axis) in the spleen. (B) Histograms show representative CFSE dye dilution by CD45.1 congenic cells transferred into CD45.2 C57BL/6 control (WT), FL−/−, and B16-FL–injected mice evaluated in the spleen at the indicated times after transfer (top). Numbers indicate percentages of cells that had undergone more than five divisions. (C) Graph shows absolute numbers ± SEM of splenic CD45.1+ donor T reg cells that had undergone zero, one to five, or more than five divisions 12 d after transfer into the indicated mice. Bar graphs in B and C summarize data (means ± SD; n = 4–6 mice in two independent experiments). (D) Dot plots show numbers of FLr+/+ CD45.1 (host) and FLr−/− CD45.1 (donor) T reg cells in mice from day −4 to day 12 of treatment with FL or PBS. Bar graphs summarize the percentage and absolute number of donor and recipient T reg cells. Histograms show CFSE dilution in splenic FLr−/− donor T reg cells 12 d after transfer (right; n = 3 mice per group in two experiments. p-values were determined by a Student's t test comparing the experimental group versus WT. *, P < 0.05; **, P < 0.01; ***, P < 0.001. ns, nonstatistically significant.
DC and T reg cell proliferation in vivo
To determine whether the increase in T reg cells in FL-treated mice was caused by the conversion of Foxp3− cells to T reg cells (Apostolou and von Boehmer, 2004), we purified allotype-marked GFP− T cells from FOXP3–diphtheria toxin receptor (DTR)–GFP mice (Kim et al., 2007) and adoptively transferred them to FL-treated recipients. Although we did find the expected increase of host T reg cells in response to FL, there was no detectable conversion of Foxp3− cells to T reg cells (Fig. S1 B). To ascertain whether the increase in T reg cells in response to FL was caused by T reg cell proliferation, we monitored T reg cell division using CFSE dye dilution. Allotype-marked, CFSE-labeled T cells were adoptively transferred into WT, FL−/−, or FL-overexpressing mice. As reported by others, autoreactive T reg cells undergo constant homeostatic division in WT mice in the steady state (Fisson et al., 2003; von Boehmer, 2003). We found that this division was impaired in DC-deficient FL−/− mice and increased in FL-overexpressing mice that had increased numbers of DCs (Fig. 1 B). In contrast, CD4+ and CD8+ T cells show little homeostatic division, and this was not altered by loss or overexpression of FL (Fig. 1 B). In addition, the absolute numbers of donor CFSE+ T reg cells that had not divided was the same irrespective of FL deficiency or overexpression. Therefore, FL did not alter these cells' survival (Fig. 1 C). To determine whether the increase in T reg cell proliferation in mice treated with FL was a direct result of the effect of FL on T reg cells, we repeated the transfer experiment using FLr-deficient (FLr−/−) donor T cells (Mackarehtschian et al., 1995). We found that FLr−/− T reg cells were similar to WT T reg cells in that they showed increased proliferation in FL-treated mice compared with uninjected controls (Fig. 1 D). We conclude that natural T reg cell proliferation in vivo increases with FL injection and decreases in its absence. However, FL does not have a direct effect on T reg cells, and therefore, FL effect is not T reg cell autonomous.
MHCII expression by DCs is required to sustain T reg cell proliferation in vivo
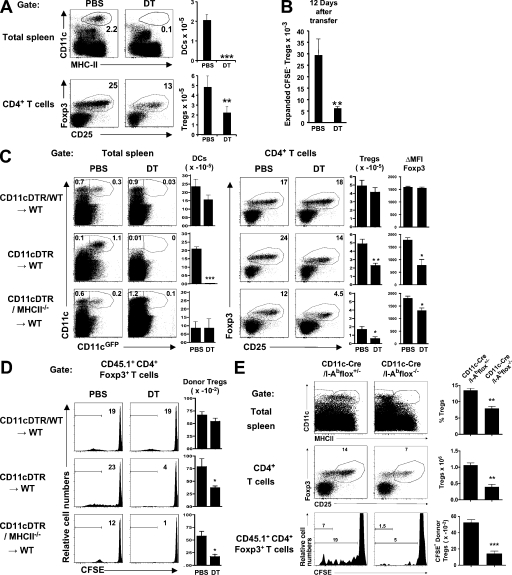
To determine whether the effects of FL were DC dependent, we ablated CD11chi DCs by administration of DT in CD11c-DTR BM chimeras. Mice treated for 6 d with DT showed a reduction in the numbers of DCs in the spleen and LN, and a two- to threefold decrease in T reg cell numbers (Fig. 2 A and Fig. S3 A). This decrease in T reg cells was not the result of a decrease in T reg cell production in the thymus (Fig. S3 B; Liston et al., 2008). In addition, adoptively transferred, CFSE-labeled, allotype-marked T reg cells showed a decrease in homeostatic division upon DC depletion (Fig. 2 B). We conclude that T reg cell homeostatic division in the LN and the spleen is dependent on the number of conventional DCs in those organs.
Figure 2.
MHCII expression by DC is required to maintain T reg cell homeostasis. (A) Dot plots show CD11c versus MHCII (top) and Foxp3 versus CD25 (bottom) staining in the spleens of CD11cDTR→WT chimeric mice after treatment with PBS or DT. Histograms show the means ± SD of the absolute number of endogenous DCs and T reg cells in spleens measured 12 d after PBS or DT treatment (n = 6 mice in one representative out of five experiments). (B) Histogram shows the mean numbers ± SD of CFSE-labeled transferred T reg cells that underwent more than five divisions in the spleens of the CD11-DTR chimeras shown in A at the indicated time points after transfer (n = 3 mice for each condition in five experiments). (C) Dot plots show endogenous DCs and T reg cells in the spleens of the indicated chimeric mice treated with PBS or DT for 6 d. Histograms summarize the number of endogenous DCs and T reg cells ± SD, and the Foxp3 intensity of expression in T reg cells as measured by the mean fluorescence index difference (ΔMFI) between T reg and CD4+Foxp3− cells ± SD (n = 2–3 mice per group in three experiments). (D) Histograms (left) show representative examples of day 7 CFSE dye dilution by transferred allotype-marked T reg cells in the experiments shown in C. Histograms (right) summarize the number of transferred CFSE-labeled T reg cells found in the spleens of the same mice (n = 2–3 mice in three experiments). (E) DCs (top) and T reg cells (middle) in the spleens of CD11c-Cre/I-Abflox+/− and CD11c-Cre/I-Abflox−/− mice, and CFSE dye dilution by allotype-marked T reg cells after 7 d (bottom). Percentages and absolute numbers of endogenous T reg cells and CFSE− donor T reg cells ± SEM are indicated (n = 3 mice per group). One representative out of two experiments is shown. p-values were determined by a Student's t test comparing the experimental group versus control. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Homeostatic T reg cell division is thought to require self-antigen presentation by MHCII because it is impaired in MHCII−/− mice (Shimoda et al., 2006). To determine whether these effects are mediated by DCs, we produced mixed BM chimeras composed of 4:1 mixtures of CD11c-GFP-DTR and WT control, or MHCII−/− cells. After DT treatment, CD11chiGFP+ cells expressing MHCII were deleted, leaving WT MHCII+CD11c+ cells in CD11cDTR/WT→WT control chimeras, but only MHCII−/−CD11c+ DCs in the CD11cDTR/MHCII−/−→WT chimeras (Fig. 2 C). In both cases, MHCII continued to be expressed by radioresistant nonhematopoietic host cells as well as by CD11c− donor cells. We found no change in T reg cell numbers after DT administration in CD11cDTR/WT→WT chimeras, whereas they were greatly reduced in CD11cDTR/MHCII−/−→WT chimeric mice (Fig. 2 C). Moreover, allotype-marked CSFE-labeled T reg cells transferred into treated CD11cDTR/MHCII−/−→WT chimeric mice showed impaired proliferation compared with controls (Fig. 2 D). In addition, we observed that the remaining T reg cells in CD11cDTR/MHCII−/−→WT DT-treated chimeras exhibit lower Foxp3 levels than their counterparts from MHCII+/+ DC-sufficient mice, as illustrated by their diminished mean fluorescence index (Fig. 2 C). A similar effect was also observed in DC-deficient DT-treated CD11cDTR→WT (Fig. 2 C) and FL−/− mice (Fig. S1 C). This feature has been associated with decreased suppressive function of T reg cells and autoimmune disorders issues (Wan and Flavell, 2007).
T reg cell proliferation in response to cultured DCs appears to be MHCII independent (Swee et al., 2009). To confirm the role of MHCII expression by DCs in T reg cell homeostasis in vivo, we deleted MHCII specifically on CD11c cells in vivo by breeding mice that carry lox-p–flanked MHC-II (I-Abflox; Hashimoto et al., 2002) with CD11c-Cre mice (Caton et al., 2007) to produce CD11c-Cre/I-Abflox mice. CD11c-Cre/I-Abflox mice lacked MHCII expression on CD11chi cells (Fig. 2 E and Fig. S3 C) and resembled FL−/− mice and CD11cDTR/MHCII−/− chimeric mice treated with DT in that they harbored fewer T reg cells than littermate controls (Fig. 2 E and Fig. S3 C). In addition, the proliferation of transferred CFSE-labeled T reg cells was impaired in CD11c-Cre/I-Abflox mice compared with controls (Fig. 2 E). We conclude that the DC-dependent feedback loop that controls T reg cell numbers and homeostatic division is dependent on MHCII expression by DCs in vivo.
Increase in Th1 and Th17 cells
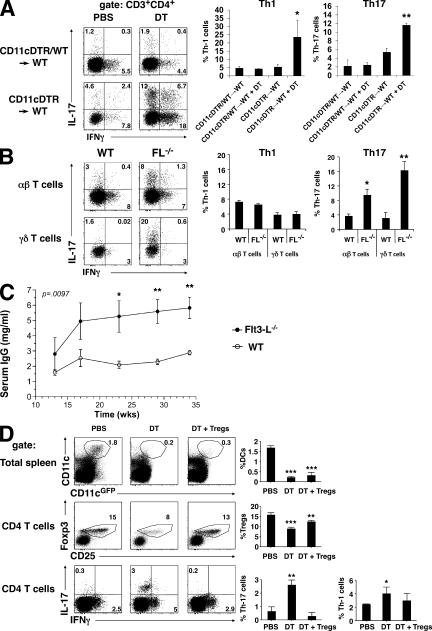
To determine whether altering T reg cell homeostasis by decreasing the number of DCs has an impact on host immune status, we measured the proportions of Th1 and Th17 cells (Korn et al., 2009) in the lamina propria of the small intestine of CD11cDTR→WT chimeric mice treated with DT. We found that IFN-γ– and IL-17–expressing cells were increased 5- and 2-fold, respectively, in the lamina propria and 10- and 2-fold, respectively, in the spleens of DT–treated CD11cDTR→WT chimeric mice but not in the CD11cDTR+WT→WT controls (Fig. 3, A and D). Similar results were obtained in DC-deficient FL−/− mice (Fig. 3 B) and CD11cDTA mice (Ohnmacht et al., 2009). In addition, we measured the levels of IgG antibodies in the serum and found that FL−/− mice developed a hypergammaglobulinemia, unlike the age-matched WT mice (Fig. 3 C). To determine whether these effects are caused by the loss of T reg cells, we complemented DC-depleted mice by injection of CD4+CD25+ regulatory T cells six hours after each DT injection. We found that injection of T reg cells prevented the increase in IL-17 in the DT-treated CD11cDTR→WT chimeric mice (Fig. 3 D). Thus, DC-deficient mice show decreased numbers of T reg cells and increased levels of autoimmune inflammation that can be reversed by T reg cells.
Figure 3.
Lack of T reg cells causes autoimmunity in DC-deficient mice. (A) Dot plots show representative examples of intracellular staining for IL-17 or IFN-γ in lamina propria CD3+CD4+ T cells from CD11cDTR→WT BM chimeras or CD11cDTR+WT→WT control mixed BM chimeras 12 d after treatment with PBS or DT. Histograms summarize the results (n = 4 experiments with two to three mice per group) and show the numbers of IFN-γ+ and IL-17+ Th cells in the lamina propria after DC depletion. p-values were calculated by a Student's t test. *, P < 0.05; **, P < 0.01. (B) Dot plots show intracellular staining for IL-17 and IFN-γ in CD3+CD4+TCRαβ T cells and CD3+TCRγδ+ T cells from the lamina propria of C57BL/6 and FL−/− mice. Histograms show the percentages of Th1 and Th17 cells among the indicated population of T cells (n = 2 mice per group in two experiments). (C) Graph shows total serum IgG levels in FL−/− (•) and B6 control (○) mice as determined by ELISA. Results are expressed as mean values ± SEM (n = 5 mice for each group). The experiment was performed twice with similar results. (D) Dot plots show CD11c versus MHCII (top), Foxp3 versus CD25 (middle), and IL-17 versus IFN-γ (bottom) expression by splenocytes (top) or CD4+ splenocytes (middle and bottom) from CD11c-DTR chimeric mice that received PBS, DT, or DT plus 8–10 × 106 T reg cells every 3 d for 12 d. Histograms show the means ± SD of the proportions of the indicated populations (n = 3 mice in two experiments). A Student's t test was used for statistical analysis. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DC-mediated increases in T reg cells prevent autoimmunity
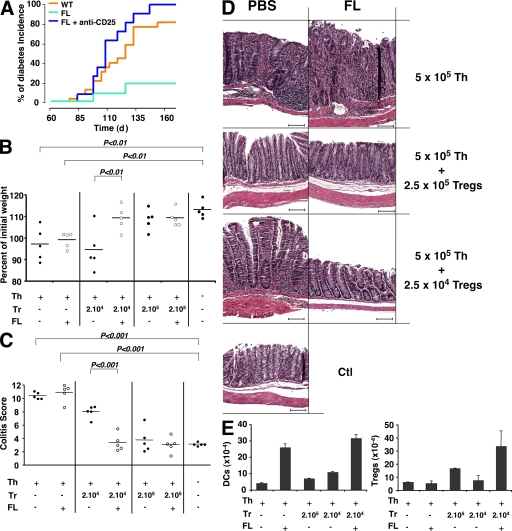
To determine whether increasing the numbers of DCs would prevent autoimmune diseases by increasing natural T reg cells, we measured the impact of increasing DC numbers on type 1 diabetes in nonobese diabetic (NOD) mice and also in a model of IBD. FL injection protects NOD mice from type 1 diabetes (O'Keeffe et al., 2005), but the question of whether this effect is mediated by T reg cells has not yet been explored. To address the question of whether T reg cells might be involved in the FL-mediated protection from type 1 diabetes, we compared FL− and FL+ anti-CD25–injected NOD mice. We found that protection from type 1 diabetes by FL in NOD mice was abrogated by anti-CD25, which suggests that the effect is T reg cell dependent (Fig. 4 A). IBD was induced by injection of naive CD4+ T cells into RAG−/− mice, leading to weight loss and gut inflammation (Powrie et al., 1993; Izcue et al., 2006). Coinjection of T reg cells protected against the disease in a dose-dependent manner (Fig. 4, B–D). For example, coinjection of 2 × 105 T reg cells with 5 × 105 CD4 T cells was protective, but 2 × 104 or 2 × 103 T reg cells was not sufficient (Fig. 4, B–D; and not depicted). FL increased the number of T reg cells in this model, but FL did not alter the course of the disease in the absence of T reg cells (Fig. 4, B–D) or in mice coinjected with 2 × 103 T reg cells (not depicted). However, the combination of FL with 2 × 104 T reg cells, which was associated with an increase in T reg cells (Fig. 4 E and Fig. S4), prevented weight loss and IBD (Fig. 4, B–D), as well as other autoimmune manifestations, such as loss of fur (Fig. S4). Thus, increasing the number of T reg cells by increasing DCs can be protective against autoimmune diseases.
Figure 4.
Effect of altered DC homeostasis on autoimmunity. (A) Protection of diabetes in FL-treated mice is abrogated by T reg cell depletion. The graph shows the incidence of diabetes over time in days in NOD mice treated with FL (green; n = 10) or PBS for 10 d (orange; n = 16; log-rank test difference, P < 0.01), or with anti-CD25 mAb injection before FL treatment (blue; n = 11 mice in two experiments). (B–E) Protection of colitis in mice treated with FL is T reg cell dependent. (B) Graph shows body weight at 16 wk relative to the initial weight of RAG−/− mice (8 wk old) that receive no cells, or 5 × 105 CD4+CD44intCD25− T cells alone or together with the indicated number of T reg cells with or without FL. Each dot represents an individual mouse (two experiments). (C) Colitis scores of the mice shown in B. The horizontal bars represent means. (D) Representative histological sections of colons stained with hematoxylin and eosin from the experiment summarized in C. Experimental groups are indicated (right), as well as injection with PBS or FL (top). Bars, 100 µm. (E) Histogram shows the mean absolute numbers ± SD of CD11chi MHCII+ DCs and CD4+CD25+Foxp3+ T reg cells in spleens from the RAG−/− mice sacrificed 7 d after adoptive transfer that received the indicated cells and treatment.
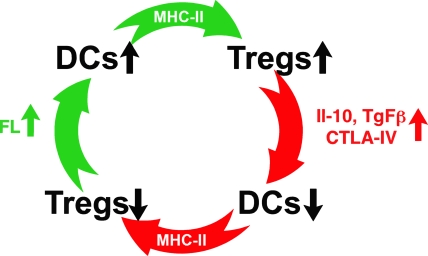
Our results reveal the existence of a previously unappreciated feedback regulatory loop that maintains the number of T reg cells and DCs in vivo. This regulatory circuit is likely to be essential to the balance between immunity and tolerance. Loss of T reg cells results in autoimmunity and an increase in the number of DCs (Kim et al., 2007; Liu et al., 2009), which is mediated in part by FLr-induced DC division in the secondary lymphoid organs (Waskow et al., 2008). The experiments reported in this paper show that the increase in DCs, in turn, results in an expansion of T reg cells by a mechanism that requires DC expression of MHCII. This feedback loop would normally result in the reestablishment of T reg cell numbers and immune balance (Fig. 5). Conversely, a loss of DCs results in a loss of T reg cells and an increased risk of autoimmune disease. However, under steady-state conditions, the decrease in T reg cells results in an FL-dependent increase in DCs, which leads to increased homeostatic expansion of T reg cells, which in turn leads to a return to immune equilibrium (Fig. 5). Although activated T cells can produce FL, the precise source of the FL that is responsible for this effect remains to be determined (Fichelson, 1998).
Figure 5.
Proposed homeostatic feedback loop between DCs and T reg cells. Increased numbers of DCs result in increased numbers of T reg cells. An increase in T reg cells leads to a decrease in DCs, which in turn decreases the number of T reg cells, which then increases DCs. MHCII expression by DCs is required for these effects. A loss of T reg cells increases DCs by a mechanism that requires FL.
Our results differ from other observations in mice in which DCs and other CD11c-expressing cells were constitutively depleted (Birnberg et al., 2008; Ohnmacht et al., 2009). In those experiments, there was a complex generalized lympho- and myeloproliferative syndrome with some features of autoimmune disease, including increased numbers of granulocytes, inflammatory mediators, and possibly T reg cells (Ohnmacht et al., 2009). This discrepancy might be attributable to the difference between acute versus chronic DC ablation in the CD11c-Cre/ROSA-DTA mice (Birnberg et al., 2008; Ohnmacht et al., 2009). The chronic model allows for the emergence of compensatory mechanisms, which can confound any primary deficits. In addition, CD11c is expressed on macrophages, NK cells, epithelial cells, and many other leukocytes; therefore, the complex immune phenotype found in CD11c-Cre/ROSA-DTA mice may be caused by effects on cells other than DCs (Birnberg et al., 2008; Ohnmacht et al., 2009). The existence of a peripheral feedback circuit between DCs and T reg cells in vivo has significant implications for immune-based therapies, as demonstrated by the effects of FL injection on the course of autoimmune diseases such as type 1 diabetes and IBD.
MATERIALS AND METHODS
Mice, FL injection, oral gavage, and cell lines.
FL−/− and FLr−/− mice were provided by I. Lemischka (The Black Family Stem Cell Institute at Mount Sinai School of Medicine, New York, NY; Mackarehtschian et al., 1995) and H.J. McKenna (Fred Hutchinson Cancer Research Center, Seattle, WA; McKenna et al., 2000), respectively. Mice carrying a transgene encoding the DTR-GFP fusion protein under the control of the mouse CD11c promoter (B6.FVB-Tg Itgax-DTR/GFP 57Lan/J or CD11c-DTR-GFP mice; a gift of S. Jung, The Weizmann Institute of Science, Rehovot, Israel) and Foxp3DTR-GFP mice that allow inducible ablation of DCs and T reg cells in vivo have been previously described (Jung et al., 2002; Kim et al., 2007). B cell–deficient mice (JH−/− mice; Chen et al., 1993)) were a gift from J. Ravetch (The Rockefeller University, New York, NY). CD11c-Cre mice (Caton et al., 2007) were a gift from B. Reizis (Columbia University Medical Center, New York, NY). I-Abflox mice have been previously described (iabneo/neo mice; Hashimoto et al., 2002). C57BL/6, CD45.1+ (SJL), B6.RAG−/−, B6-MHCII−/−, and NOD mice came either from the Jackson Laboratory or were bred at the Rockefeller University. All mice were maintained in specific pathogen-free conditions. Mice compared in each experiment were from the same commercial origin and were cohoused to minimize the influence of gut microflora (Ivanov et al., 2008). The experiments were conducted according to Rockefeller University Animal Care and Use Committee–approved protocols. rhFL was obtained from Amgen. rmFL was produced by anti-Flag–hemagglutinin (HA) purification of supernatant from Chinese hamster ovary (CHO) cells expressing rmFLt-3L-FlagHA (a gift from C.G. Park, The Rockefeller University, New York, NY). The B16-FL tumor cell line was obtained from G. Dranoff (Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA; Mach et al., 2000) and cultured in DMEM (Invitrogen) complemented with 10% FCS, 2 mM l-glutamine (Invitrogen), and 100 U/ml penicillin-streptomycin (Invitrogen). 106 tumor cells were injected subcutaneously. All cell lines were mycoplasma free. Mice were gavaged with 40 mg/kg sunitinib (Sutent; Pfizer) via feeding needles (Popper) once daily during the first 10 d of treatment and thereafter every 2 d.
Antibodies and flow cytometry analyses.
The following mAbs were used: anti-CD3 (2C11), CD4 (RM4-5), CD8 (53-6.7), CD11c (HL3), CD19 (1D3), CD25 (PC-61 or 7D4), CD44 (IM7), CD45.1 (A20), CD45.2 (104), CD45R (RA3-6B2), CD135 (A2F10), MHCII (M5/114.15.2; from either BD or eBioscience), and PDCA1 (JFOS-1C2.4.1; Miltenyi Biotec). Intracellular labeling of the transcription factor Foxp3 by anti-Foxp3 antibody (FJK-16s; e-Bioscience) was performed according to the manufacturer's recommendations. Isotype-irrelevant mAbs were used as controls. Processed cells were analyzed with a flow cytometer (LSR II; BD). Further analyses were performed with FlowJo software (Tree Star, Inc.). After adoptive transfer in WT hosts under our experimental conditions (Fisson et al., 2003), donor T reg cells represented 0.1% of splenocytes. Therefore, 2–5 × 106 events were acquired for each analysis.
CFSE staining and adoptive transfer of cells.
MACS-sorted (Miltenyi Biotec) CD4+CD25+ or CD4+CD25− T cells or unfractionated cells from LN and spleens were labeled with CFSE (Sigma-Aldrich), as previously described (Fisson et al., 2003), and transferred intravenously. Transferred cells were identified based on CD4, Foxp3, and CD45.1/2 expression.
DT-induced DC depletion in chimeric mice.
DC depletion was performed in CD11c-DTR BM chimeras. Recipient mice were irradiated with 550 cGy plus 500 cGy, with 3 h between doses, and 8–16 × 106 BM cells were injected intravenously 3 h later. In the case of mixed chimeras, we used a 1:4 ratio of CD11c-DTR/C57BL/6 or MHCII−/− BM. Mice were fed with antibiotic-supplemented food (TestDiet) and were given free access to water. Mice were used for experiments 8 wk after reconstitution. For DC depletion, recipients received injections of 1 µg DT from Corynebacterium diphtheriae (Sigma-Aldrich) intraperitoneally every 2–3 d for 6–18 d.
Isolation of lamina propria lymphocytes.
Intestines were collected and flushed with ice-cold PBS, and Peyer's patches were removed. Intestines were opened longitudinally and cut into 2–3-cm pieces. The pieces were washed once with cold PBS plus 1mM dithiothreitol and incubated twice in 10 ml of 30 mM EDTA, 10 mM Hepes in PBS for 10 min at 37°C under slow rotation (150 rpm). The remaining tissue was washed once in complete RPMI 1640 medium (Invitrogen) supplemented with 10% FCS, and digested at 37°C for 90 min in complete RPMI 1640 containing collagenase (Sigma-Aldrich) and 150 µg/ml DNase I (Roche). Digested tissue was passed through a 70-µm cell strainer. The flow-through was pelleted and subjected to a 40:80% Percoll gradient under centrifugation for 20 min at 2,500 rpm at room temperature. Lamina propria leukocytes were collected at the interphase of the Percoll gradient, washed once, and resuspended in FACS buffer or complete RPMI 1640 medium. As lamina propria cell recovery from inflamed tissue is less efficient than from healthy intestine, only a proportion of different immune cells and not absolute numbers were assessed.
Intracellular cytokine staining.
Cells obtained from the dissection of mesenteric LNs or lamina propria were incubated at 37°C for 7 h in 10%FCS complete RPMI 1640 medium with 50 ng/ml PMA (Sigma-Aldrich), 750 ng/ml ionomycin (Sigma-Aldrich), and 1 µl/ml GolgiPlug (BD). Surface staining was performed for 30 min, after which the cells were resuspended in fixation/permeabilization solution (Cytofix/Cytoperm kit; BD). Intracellular cytokine staining using anti–IFN-γ and anti–IL-17 (BD) was performed according to the manufacturer's protocol.
T reg cell depletion.
T reg cells were depleted by intraperitoneal injection of 250 mg anti-CD25 mAb (clone PC61; produced by the Memorial Sloan-Kettering Cancer Center/Rockefeller University monoclonal antibody core facility; Darrasse-Jèze et al., 2006).
Assessment of diabetes.
Blood glucose levels were measured every week with a glucose meter (One Touch II; Lifescan). Mice were considered diabetic after three consecutive measurements >200 mg/dl. The onset of diabetes was dated from the first of the sequential diabetic measurements.
IBD model.
LN and spleen CD4 T cells from C57BL/6 mice were purified by MACS using a CD4+ T cell isolation kit (Miltenyi Biotec). CD4+CD25hi (T reg) and CD4+CD25−CD44int (naive Th) cells were purified by cell sorting (BD; Fisson et al., 2003). 4 × 105 naive Th cells were injected alone or together with variable numbers of T reg cells. Weights were measured every week, and mice were sacrificed for histological evaluation of colitis, as previously described (Read and Powrie, 2001; Izcue et al., 2008).
Statistical methods.
Nonlinear regression curves were determined using Prism software (GraphPad Software, Inc.). Statistical analyses of the survival curves were performed using a log-rank test. Comparisons of one-variable data were performed using a two-tailed unpaired Student's t test. Comparisons of two-variable data were performed using two-way analysis of variance tests with the Bonferroni adjustment. We evaluated statistical significance with Prism software. Data are expressed as means ± SD. P < 0.05 was considered significant.
Online supplemental material.
Fig. S1 describes T reg cell homeostasis in mice overexpressing or deficient for FL, the correlation of DC and T reg cell levels in the LN, the absence of converstion of CD4+CD25+ T cells to Foxp3+CD25+ T reg cells by treatment with FL, and Foxp3 expression in splenic T reg cells from FL−/− mice. Fig. S2 demonstrates that B cell–deficient mice exhibit normal proportions and numbers of T reg cell splenocytes. Fig. S3 describes T reg cell homeostasis in lymphoid organs of CD11c-DTR and CD11c-Cre/I-Abflox−/− mice. Fig. S4 shows that FL treatment of T cell–transferred RAG−/− mice increases T reg cell levels and protects them from hair loss and dermatitis. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20090746/DC1.
Acknowledgments
We thank O. Turovskaya for her precious help with IBD scoring, I. Lemischka for Flt3−/− mice, H.J. McKenna for FL−/− mice, S. Jung for CD11c-DTR mice, B. Reizis for CD11c-Cre mice, J. Ravetch for Jh−/− mice, C.G. Park for the CHO-FL cell line, G. Dranoff for the B16-FL cell line, K. Velinzon for cell sorting, R. Steinman and members of the Nussenzweig laboratory for discussions and critical reading of the manuscript, P. Ahern for technical advice, D. Bosque for technical help, and S. Davis for editorial assistance.
G. Darrasse-Jèze was supported by a Human Frontier long-term fellowship (LT00291/2008). K. Liu was supported by a C.H. Li Memorial Scholarship Award from the Rockefeller University. This work was supported in part by grant AI051573 from the National Institutes of Health to M.C. Nussenzweig. A. Rudensky and M.C. Nussenzweig are Howard Hughes Medical Institute investigators.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used: DT, diphtheria toxin; DTR, DT receptor; FL, Flt3 ligand; FLr, Flt3 receptor; IBD: inflammatory bowel disease; NOD, nonobese diabetic.
References
- Apostolou I., von Boehmer H. 2004. In vivo instruction of suppressor commitment in naive T cells.J. Exp. Med. 199:1401–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J., Steinman R.M. 1998. Dendritic cells and the control of immunity.Nature. 392:245–252 [DOI] [PubMed] [Google Scholar]
- Birnberg T., Bar-On L., Sapoznikov A., Caton M.L., Cervantes-Barragán L., Makia D., Krauthgamer R., Brenner O., Ludewig B., Brockschnieder D., et al. 2008. Lack of conventional dendritic cells is compatible with normal development and T cell homeostasis, but causes myeloid proliferative syndrome.Immunity. 29:986–997 [DOI] [PubMed] [Google Scholar]
- Brocker T., Riedinger M., Karjalainen K. 1997. Targeted expression of major histocompatibility complex (MHC) class II molecules demonstrates that dendritic cells can induce negative but not positive selection of thymocytes in vivo.J. Exp. Med. 185:541–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton M.L., Smith-Raska M.R., Reizis B. 2007. Notch–RBP-J signaling controls the homeostasis of CD8− dendritic cells in the spleen.J. Exp. Med. 204:1653–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Trounstine M., Alt F.W., Young F., Kurahara C., Loring J.F., Huszar D. 1993. Immunoglobulin gene rearrangement in B cell deficient mice generated by targeted deletion of the JH locus.Int. Immunol. 5:647–656 [DOI] [PubMed] [Google Scholar]
- Darrasse-Jèze G., Klatzmann D., Charlotte F., Salomon B.L., Cohen J.L. 2006. CD4+CD25+ regulatory/suppressor T cells prevent allogeneic fetus rejection in mice.Immunol. Lett. 102:106–109 [DOI] [PubMed] [Google Scholar]
- Denning T.L., Wang Y.C., Patel S.R., Williams I.R., Pulendran B. 2007. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses.Nat. Immunol. 8:1086–1094 [DOI] [PubMed] [Google Scholar]
- Fichelson S. 1998. The FLT3/FLK2 ligand: structure, functions and prospects.Eur. Cytokine Netw. 9:7–22 [PubMed] [Google Scholar]
- Fisson S., Darrasse-Jèze G., Litvinova E., Septier F., Klatzmann D., Liblau R., Salomon B.L. 2003. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state.J. Exp. Med. 198:737–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K., Joshi S.K., Koni P.A. 2002. A conditional null allele of the major histocompatibility IA-beta chain gene.Genesis. 32:152–153 [DOI] [PubMed] [Google Scholar]
- Hawiger D., Inaba K., Dorsett Y., Guo M., Mahnke K., Rivera M., Ravetch J.V., Steinman R.M., Nussenzweig M.C. 2001. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo.J. Exp. Med. 194:769–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawiger D., Masilamani R.F., Bettelli E., Kuchroo V.K., Nussenzweig M.C. 2004. Immunological unresponsiveness characterized by increased expression of CD5 on peripheral T cells induced by dendritic cells in vivo.Immunity. 20:695–705 [DOI] [PubMed] [Google Scholar]
- Ivanov I.I., Frutos Rde.L., Manel N., Yoshinaga K., Rifkin D.B., Sartor R.B., Finlay B.B., Littman D.R. 2008. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine.Cell Host Microbe. 4:337–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izcue A., Coombes J.L., Powrie F. 2006. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation.Immunol. Rev. 212:256–271 [DOI] [PubMed] [Google Scholar]
- Izcue A., Hue S., Buonocore S., Arancibia-Cárcamo C.V., Ahern P.P., Iwakura Y., Maloy K.J., Powrie F. 2008. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis.Immunity. 28:559–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S., Unutmaz D., Wong P., Sano G., De los Santos K., Sparwasser T., Wu S., Vuthoori S., Ko K., Zavala F., et al. 2002. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens.Immunity. 17:211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.M., Rasmussen J.P., Rudensky A.Y. 2007. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice.Nat. Immunol. 8:191–197 [DOI] [PubMed] [Google Scholar]
- Klein L., Khazaie K., von Boehmer H. 2003. In vivo dynamics of antigen-specific regulatory T cells not predicted from behavior in vitro.Proc. Natl. Acad. Sci. USA. 100:8886–8891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T., Anderson A.C., Bettelli E., Oukka M. 2007. The dynamics of effector T cells and Foxp3+ regulatory T cells in the promotion and regulation of autoimmune encephalomyelitis.J. Neuroimmunol. 191:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T., Bettelli E., Oukka M., Kuchroo V.K. 2009. IL-17 and Th17 cells.Annu. Rev. Immunol. 27:485–517 [DOI] [PubMed] [Google Scholar]
- Kretschmer K., Apostolou I., Hawiger D., Khazaie K., Nussenzweig M.C., von Boehmer H. 2005. Inducing and expanding regulatory T cell populations by foreign antigen.Nat. Immunol. 6:1219–1227 [DOI] [PubMed] [Google Scholar]
- Kronenberg M., Rudensky A. 2005. Regulation of immunity by self-reactive T cells.Nature. 435:598–604 [DOI] [PubMed] [Google Scholar]
- Liston A., Nutsch K.M., Farr A.G., Lund J.M., Rasmussen J.P., Koni P.A., Rudensky A.Y. 2008. Differentiation of regulatory Foxp3+ T cells in the thymic cortex.Proc. Natl. Acad. Sci. USA. 105:11903–11908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Victora G.D., Schwickert T.A., Guermonprez P., Meredith M.M., Yao K., Chu F.F., Randolph G.J., Rudensky A.Y., Nussenzweig M. 2009. In vivo analysis of dendritic cell development and homeostasis.Science. 324:392–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckashenak N., Schroeder S., Endt K., Schmidt D., Mahnke K., Bachmann M.F., Marconi P., Deeg C.A., Brocker T. 2008. Constitutive crosspresentation of tissue antigens by dendritic cells controls CD8+ T cell tolerance in vivo.Immunity. 28:521–532 [DOI] [PubMed] [Google Scholar]
- Mach N., Gillessen S., Wilson S.B., Sheehan C., Mihm M., Dranoff G. 2000. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand.Cancer Res. 60:3239–3246 [PubMed] [Google Scholar]
- Mackarehtschian K., Hardin J.D., Moore K.A., Boast S., Goff S.P., Lemischka I.R. 1995. Targeted disruption of the flk2/flt3 gene leads to deficiencies in primitive hematopoietic progenitors.Immunity. 3:147–161 [DOI] [PubMed] [Google Scholar]
- Maraskovsky E., Brasel K., Teepe M., Roux E.R., Lyman S.D., Shortman K., McKenna H.J. 1996. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified.J. Exp. Med. 184:1953–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna H.J., Stocking K.L., Miller R.E., Brasel K., De Smedt T., Maraskovsky E., Maliszewski C.R., Lynch D.H., Smith J., Pulendran B., et al. 2000. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells.Blood. 95:3489–3497 [PubMed] [Google Scholar]
- O'Farrell A.M., Abrams T.J., Yuen H.A., Ngai T.J., Louie S.G., Yee K.W., Wong L.M., Hong W., Lee L.B., Town A., et al. 2003. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo.Blood. 101:3597–3605 [DOI] [PubMed] [Google Scholar]
- O'Keeffe M., Brodnicki T.C., Fancke B., Vremec D., Morahan G., Maraskovsky E., Steptoe R., Harrison L.C., Shortman K. 2005. Fms-like tyrosine kinase 3 ligand administration overcomes a genetically determined dendritic cell deficiency in NOD mice and protects against diabetes development.Int. Immunol. 17:307–314 [DOI] [PubMed] [Google Scholar]
- Ohnmacht C., Pullner A., King S.B., Drexler I., Meier S., Brocker T., Voehringer D. 2009. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity.J. Exp. Med. 206:549–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F., Leach M.W., Mauze S., Caddle L.B., Coffman R.L. 1993. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice.Int. Immunol. 5:1461–1471 [DOI] [PubMed] [Google Scholar]
- Read S., Powrie F. 2001. Induction of inflammatory bowel disease in immunodeficient mice by depletion of regulatory T cells.Curr. Protoc. Immunol. Chapter 15:Unit 15.13 [DOI] [PubMed] [Google Scholar]
- Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases.J. Immunol. 155:1151–1164 [PubMed] [Google Scholar]
- Sakaguchi S., Yamaguchi T., Nomura T., Ono M. 2008. Regulatory T cells and immune tolerance.Cell. 133:775–787 [DOI] [PubMed] [Google Scholar]
- Salomon B., Lenschow D.J., Rhee L., Ashourian N., Singh B., Sharpe A., Bluestone J.A. 2000. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes.Immunity. 12:431–440 [DOI] [PubMed] [Google Scholar]
- Samy E.T., Parker L.A., Sharp C.P., Tung K.S. 2005. Continuous control of autoimmune disease by antigen-dependent polyclonal CD4+CD25+ regulatory T cells in the regional lymph node.J. Exp. Med. 202:771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setoguchi R., Hori S., Takahashi T., Sakaguchi S. 2005. Homeostatic maintenance of natural Foxp3+ CD25+ CD4+ regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization.J. Exp. Med. 201:723–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda M., Mmanywa F., Joshi S.K., Li T., Miyake K., Pihkala J., Abbas J.A., Koni P.A. 2006. Conditional ablation of MHC-II suggests an indirect role for MHC-II in regulatory CD4 T cell maintenance.J. Immunol. 176:6503–6511 [DOI] [PubMed] [Google Scholar]
- Steinman R.M., Nussenzweig M.C. 2002. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance.Proc. Natl. Acad. Sci. USA. 99:351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swee L.K., Bosco N., Malissen B., Ceredig R., Rolink A. 2009. Expansion of peripheral naturally occurring T regulatory cells by Fms-like tyrosine kinase 3 ligand treatment.Blood. 113:6277–6287 [DOI] [PubMed] [Google Scholar]
- Tarbell K.V., Yamazaki S., Olson K., Toy P., Steinman R.M. 2004. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes.J. Exp. Med. 199:1467–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton A.M., Shevach E.M. 1998. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production.J. Exp. Med. 188:287–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer H. 2003. Dynamics of suppressor T cells: in vivo veritas.J. Exp. Med. 198:845–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y.Y., Flavell R.A. 2007. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression.Nature. 445:766–770 [DOI] [PubMed] [Google Scholar]
- Waskow C., Liu K., Darrasse-Jèze G., Guermonprez P., Ginhoux F., Merad M., Shengelia T., Yao K., Nussenzweig M. 2008. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues.Nat. Immunol. 9:676–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S., Dudziak D., Heidkamp G.F., Fiorese C., Bonito A.J., Inaba K., Nussenzweig M.C., Steinman R.M. 2008. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells.J. Immunol. 181:6923–6933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You S., Alyanakian M.A., Segovia B., Damotte D., Bluestone J., Bach J.F., Chatenoud L. 2008. Immunoregulatory pathways controlling progression of autoimmunity in NOD mice.Ann. NY Acad. Sci. 1150:300–310 [DOI] [PMC free article] [PubMed] [Google Scholar]