Figure 1.
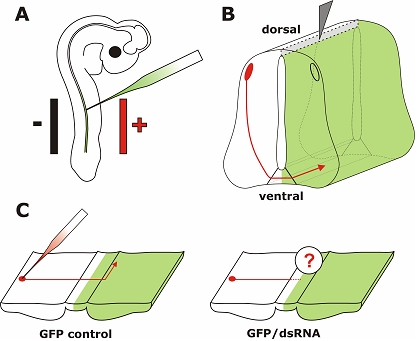
In ovo RNAi is an efficient method to silence genes in a temporally and spatially controlled manner in the developing neural tube. The chicken embryo can be accessed through a window cut into the eggshell. Phosphate-buffered saline containing long dsRNA, siRNA, or a plasmid encoding shRNA and 0.04% Trypan Blue is injected into the neural tube of the developing embryo with a glass pipette (A). In order to visualize the area where the injected RNA was taken up or as a control a plasmid encoding GFP can be co-injected. Wire electrodes are positioned parallel to the longitudinal axis of the embryo. Due to the negative charge of the nucleic acids cells toward the side of the anode are transfected. On average, we achieved 60% transfection efficiency in the targeted area of the neural tube of a 3-day-old embryo using 5 pulses of 25 Volts and of 50 msec duration (Pekarik et al, 2003). We use a one second inter-pulse interval. Depending on the position of the electrodes the target area can be selected. Positioning the electrodes dorsally will only target dorsal cell of the developing neural tube, whereas a more ventral position will result in transfected cells all along the dorso-ventral axis (as shown in B). To analyze the resulting phenotypes induced by knockdown of the target gene a variety of methods can be used. As an example we illustrate visualizing the trajectory of dorsolateral commissural neurons. These neurons extend their axons toward the floor plate, the structure that forms the ventral midline of the spinal cord. Axons cross the ventral midline before turning rostrally along the contralateral side of the floor plate (Bourikas et al, 2005a). The trajectory of these axons can be visualized by application of the lipophilic dye DiI to the cell bodies of commissural neurons (C). The comparison between control embryos, injected with a plasmid encoding GFP only or a control siRNA, with embryos injected with the target-specific dsRNA would reveal phenotypes in axon pathfinding. In the situation shown here, these would be caused by cell non-autonomous functions of the target gene as commissural axons from the side contralateral to the electroporated area are traced. For detailed protocols see references in Table 2.
