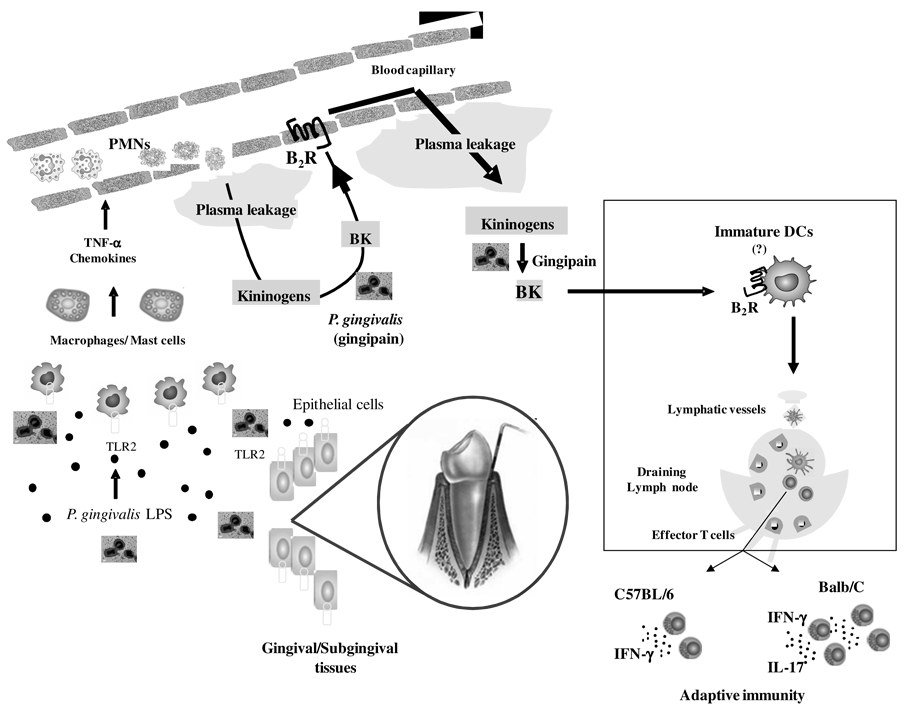
Figure 9. Trans-cellular TLR2/B2R “cross-talk” drives proteolytic generation of immunostimulatory bradykinin in extravascular mucosal tissues.
At the left side of the panel, P. gingivalis W83 (injected in the bottom of mandibular anterior vestibule) stimulate TLR2 expressed by hitherto uncharacterized resident sentinel cells (e.g., gingival epithelial cells, macrophages, mast cells). This innate recognition response translates into secretion of chemokines/cytokines, which then rapidly amplify inflammation through the activation of neutrophils/endothelium. Owing to increased vascular permeability, plasma proteins extravasate, leading to the accumulation of blood-borne kininogens in extravascular tissues (center of panel). Further downstream, the cysteine protease gingipain proteolytically liberates the vasoactive kinin moiety from kininogens. Plasma leakage is fueled through reiterative cycles of B2R-dependent triggering of the endothelium by the vasoactive kinins. Based on analysis of the immunological roles of kinins released by T. cruzi (46, 48, 49), we hypothesize that immature (myeloid) DCs patrolling the inflamed mucosa (see rectangle) are activated via the kinin/B2R pathway. After migrating to submandibular draining lymph nodes, the fimbriae-bearing DCs prime naïve T cells, while at the same time instructing their development into IFN-γ-producing effector T cells (in Balb/C and C57BL/6 mice) and IL-17-producing T cells (Balb/C mice).