Figure 2.
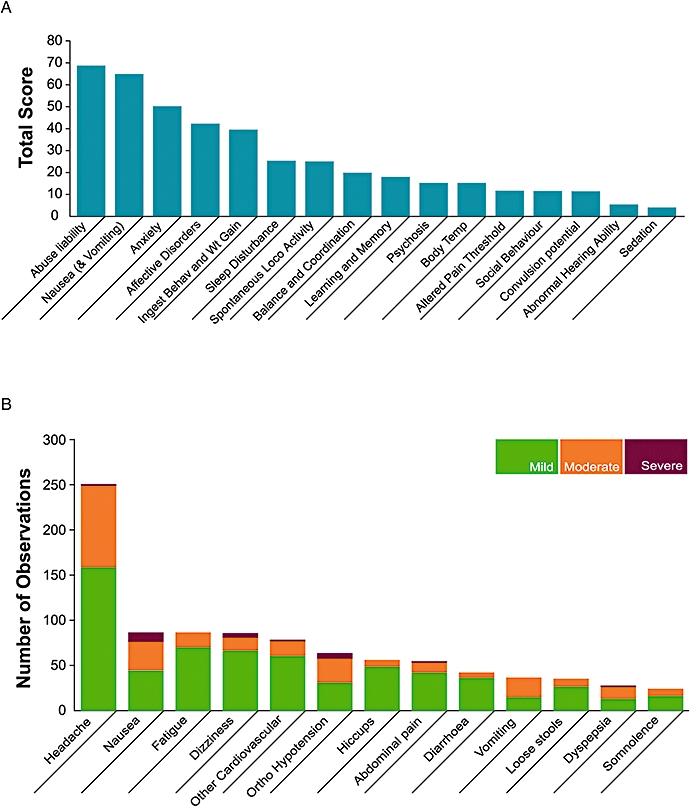
The impact of nausea and vomiting on the development of novel chemical entities. (A) An analysis by Pfizer assessing how various side effects encountered in preclinical safety studies impacted on the development of a medicine for humans. More than 70 novel therapeutics, including antivirals, agents to promote tissue repair, neurology, sex health, allergy and respiratory, cardiovascular, gastrointestinal and urogenital disease targets were used in this analysis. The targets included were agents with both peripheral and central sites of action and were assessed during the period 1998–2000. The total score represents the number of compounds progressing to clinical development that interact with targets that have known or suspected safety liability based on experimental data or from literature reports. This analysis took into account not only the impact to the patients but also to the drug development programme through the need for additional studies to investigate the extent and seriousness of the safety issue and also to support regulatory acceptance by bodies such as the European Medicines Agency and the US Food and Drug Administration. Whereas factors such as sedation, convulsion potential and changes in body temperature were important, they could be easily examined both clinically and preclinically. In contrast nausea and vomiting were considered second only to abuse liability as having an impact on the development of the drug. (B) A further analysis of side effects encountered in 16 phase 1 clinical studies conducted by Pfizer between 2003 and 2005. While the most commonly encountered side effect was headache, with approximately 250 instances, the next most encountered was nausea, which accounted for over 80 instances, nearly half of which were rated as either moderate or severe. There were also a similar number of observations of moderate vomiting. The 16 trials averaged approximately 35 individuals each.
