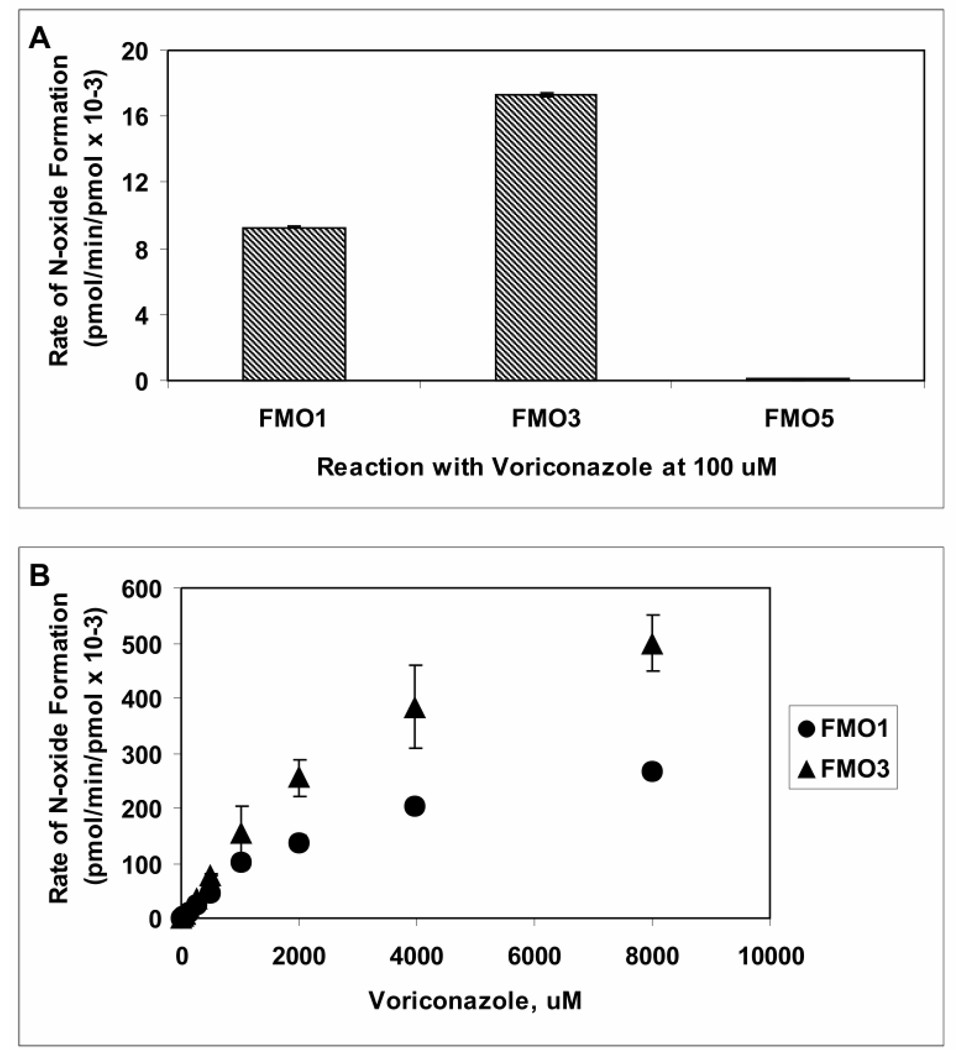
Figure 6. Catalytic Activities of Recombinant Human Flavin-containing Monooxygenase 1, 3, and 5 toward Voriconazole.
A- Voriconazole (100 µM) was metabolized by recombinant human FMO enzymes FMO1 (60 pmol/mL), FMO3 (60 pmol/mL) and FMO5 (150 pmol/mL) in presence of NADPH for 60 minutes, and rates of metabolism (pmol/min/pmol enzyme) were calculated from a calibration curve constructed using the authentic N-oxide standard. B- Initial velocity of voriconazole N-oxide formation by FMO1 (triangle) and FMO3 (circle) was determined as a function of voriconazole concentration; data are reported as mean (n=3) ± S.D. The N-oxide formation by FMO5, was undetectable.