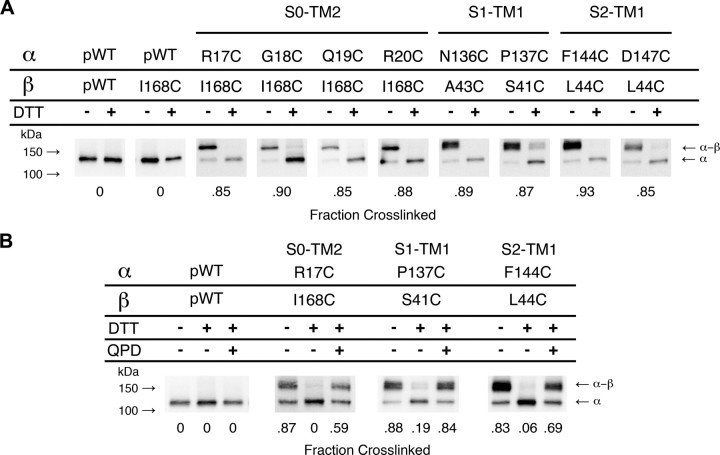
Figure 2.
Detecting disulfide crosslinking between BK α and β4 subunits. A, Western blots illustrating endogenous crosslinking of Cys-substituted α and Cys-substituted β4. Expression of mutants, selection of channels transported to the cell surface, SDS gel electrophoresis, and blotting are described in Materials and Methods. The blots were developed with an antibody against an epitope from the C terminus of α, which recognizes α (125 kDa) and the crosslinked α–β4 complex (∼155 kDa). Half of each sample was reduced with DTT (+DTT lanes). Under each gel is the fraction of crosslinked α–β4 complex in the unreduced lane. B, Reduction and reoxidation of the disulfide between R17C and I168C, P137C and S41C, and F144C and L44C. Channels on intact cells were reduced with DTT and oxidized for 20 min with 40 μm QPD. At the bottom of each lane is the fraction of crosslinked α–β4 complex.