Abstract
Background
Asthmatics often report the triggering or exacerbation of respiratory symptoms following exposure to airborne irritants, which in some cases may result from stimulation of irritant receptors in the upper airways inducing reflexive broncho-constriction. Ammonia (NH3) is a common constituent of commercially available household products, and in high concentration has the potential to elicit sensory irritation in the eyes and upper respiratory tract of humans. The goal of the present study was to evaluate the irritation potential of ammonia in asthmatics and healthy volunteers and to determine whether differences in nasal or ocular irritant sensitivity to ammonia between these two groups could account for the exacerbation of symptoms reported by asthmatics following exposure to an irritant.
Methods
25 healthy and 15 mild/moderate persistent asthmatic volunteers, with reported sensitivity to household cleaning products, were evaluated for their sensitivity to the ocular and nasal irritancy of NH3. Lung function was evaluated at baseline and multiple time points following exposure.
Results
Irritation thresholds did not differ between asthmatics and healthy controls, nor did ratings of odor intensity, annoyance and irritancy following exposure to NH3 concentrations at and above the irritant threshold for longer periods of time (30 sec).Importantly, no changes in lung function occurred following exposure to NH3 for any individuals in either group.
Conclusion
Despite heightened symptom reports to environmental irritants among asthmatics, the ocular and nasal trigeminal system of mild-moderate asthmatics does not appear to be more sensitive or more reactive than that of non-asthmatics, nor does short duration exposure to ammonia at irritant levels induce changes in lung function. At least in brief exposures, the basis for some asthmatics to experience adverse responses to volatile compounds in everyday life may arise from factors other than trigeminally-mediated reflexes.
Keywords: Asthma, trigeminal, sensory irritation, ammonia, naso-bronchial reflex, lateralization, inflammation
Introduction
Many volatile organic compounds in ambient air are capable of producing the sensation of odor and at higher concentrations, evoking trigeminally-mediated irritation of the eyes, nose and throat. This constellation of irritation symptoms is one of the dominant complaints in many indoor environments. The distribution of symptom reports ostensibly related to the presence of these compounds is somewhat heterogenous, with females, younger individuals and individuals with allergic rhinitis and respiratory problems exhibiting a heightened tendency to report symptoms (Shusterman 2007). At least some of the symptom reports appear to have an objective basis: In studies evaluating nasal sensitivity to irritants such as acetic acid or CO2, individuals with allergic rhinitis detected the onset of nasal irritation at lower concentrations than did non-rhinitic individuals (Shusterman et al. 2005;Shusterman et al. 2003b). This heightened sensitivity to irritants was attributed to the neurogenic reflexes triggered by chronic nasal inflammation.
Sensitive subpopulations, such as individuals with asthma and other respiratory diseases often report feeling ‘tightness in the chest’, ‘breathlessness’ or ‘wheezing’ after exposure to airborne compounds capable of eliciting upper airway irritation, such as those found in household cleaners, bleach or strong perfume. Although many of these compounds do not directly affect the lower airways, the afferents of the nasal trigeminal nerve have been shown to induce reflex broncho-constriction following exposure to various types of stimulation (Widdicombe 1986), including cold air (Johansson et al. 2000), capsaicin (Plevkova et al. 2004) and some irritant chemicals (Togias 2003). Thus, experiencing nasal stimulation of the trigeminal nerve may be sufficient in some asthmatics to induce asthma symptoms. If the sensitivity of the nasal and ocular trigeminal system among asthmatics is lower than for comparable healthy controls, this could provide an additional basis for the adverse reactions experienced by this sensitive subpopulation upon exposure to many household and consumer products.
The goals of this study were to determine (1) if the upper airways and eyes of asthmatics were indeed more sensitive to an irritant compound than those of non-asthmatic individuals and (2) whether brief exposures to an irritant would induce naso-bronchial reflexes and result in a change in airway function or symptom reports among this group. Ammonia was chosen as the irritant compound to study due to its widespread use in household cleaning products and the ubiquitous potential for exposure
Method
Subjects
25 healthy (age 29.7 ± 10.8) and 15 mild-moderate asthmatic individuals (age 29.1±9.6) participated in this study. They were recruited through advertisements posted at the Monell Chemical Senses Center, and in newspapers with circulation throughout Philadelphia area. Volunteers who responded to the advertisements were given a medical screening form to complete, which assessed their overall health, smoking habits, medication use, occupational and non-occupational chemical exposure history. Any individuals found to have the following medical conditions, neurologic impairment or related condition(s) were excluded from further participation: chronic sinus infection, allergic or non-allergic rhinitis (controls only), ongoing cold/flu, emphysema, chronic coughing, severe persistent asthma, chronic or recurring lung infection, active seasonal allergies, seizure disorders, Parkinson’s disease, Kallman syndrome, familial dysautonomia, Bell’s palsy, Alzheimer’s disease, multiple sclerosis, cardiovascular disease, current drug or alcohol abuse, and pregnancy or lactation. Participants were also asked to fill out the chemical intolerance inventory, a ten item questionnaire evaluating adverse responses from exposure to a number of environmental agents (i.e. paints, cleaning products, fragrances).
Subjects with asthma were classified as either mild persistent or moderate persistent according to the NIH’s classification of severity of asthmatics (Table 1). Their medical history, information on allergies and medications currently utilized was also acquired. Baseline pulmonary function measurements, forced expiratory volume in one second (FEV1) and the ratio of FEV1 to forced vital capacity (FEV1/FVC), were assessed prior to entering the study using spirometry (Koko Legend Spirometer, Ferraris Respiratory, Louisville CO). Individuals whose FEV1/FVC ratio was less then 60% or whose FEV1 14 measurement was less than 60% of predicted, were excluded from the study (i.e. classified as severe persistent asthmatic).
Table 1.
NIH Classification of Asthma Severity
| Mild Persistent | Moderate Persistent | |
|---|---|---|
| Report symptoms | >2 times/week but<1time/day | Daily |
| Report night time symptoms | >2 times/month | >1 time/week |
| Asthma Exacerbation | Exacerbation may affect activity | Exacerbations affect activity |
| FEV1 | ≥80% of Predicted | >60% to <80% of Predicted |
| PEF variability | 20 to 30% | >30% |
FEV1 = Forced expiratory volume in one second
PEF = Peak expiratory flow
Among the asthmatic cohort, were classified as mild persistent and 5 as moderate persistent, with diagnoses having been an average of 17.8 years prior (range: 5–44 years). Only two of the participants were currently taking steroids for their asthma symptoms. However, all reported having asthma symptoms an average of 5 times/week and using a short-acting inhaler an average of 2.5 times/day. All but two reported experiencing both seasonal and perennial allergies, although the presence of allergy symptoms at the time of testing was an exclusion criterion. All but one of the asthmatics and all of the controls reported having a normal sense of smell. On the chemical intolerance inventory, where a 0 indicated never being bothered and 5 indicated always being bothered, all asthmatics reported being symptomatic to household cleaning products ranging from much of the time to all of the time (average = 3.95). In contrast, the healthy controls reported occasionally or never being bothered by cleaning products (average= .85).
Participants were given an explanation of the entire experimental procedure and asked to read and sign an informed consent document developed and approved by the Schulman Associates Institutional Review Board (Cincinnati, OH). Each participant was asked to refrain from smoking, eating or drinking for at least one hour prior to testing. Prior to the first test, all individuals were screened for normal olfactory function using the NASA Odor Screening Test, which is a 7-item olfactory evaluation method adopted from an ASTM standard method (Dalton et al. 2003)
On each day of testing, participants were asked to fill out baseline symptom reports, to ensure they were free of colds, allergies or other symptoms. In addition, each asthmatic subject completed a pre-exposure medical assessment via spirometry, a pre-exposure medical questionnaire, the Likert and ordinal symptom-ranking scales (Smeets et al. 2002) and one set of three peak flow maneuvers. The medical monitor reviewed the data and reaffirmed each participants’ eligibility before testing could proceed on that day. Experiments were conducted in specially-designed testing rooms and laboratories at the Monell Chemical Senses Center by trained Monell employees. Study participants came to Monell for 3 test sessions, at the same time of the day for testing. Each session lasted up to 2 ½ hours. Testing sessions were separated by at least 48 hours, to minimize a possible carryover effect.
Ammonia Stimuli Generation
The source gas was a specialty mix of 1000ppm NH3 in an air balance (AirGas specialty gases). Ammonia concentrations were measured and verified online and offline using a MiniRae photoionization detector (Rae Systems, CA) and an Agilent 6890 gas chromatograph. Ammonia vapor was delivered via an interface with an air-dilution olfactometer at a flow rate of 500 ml/ min for the single threshold or 250 ml/min for the combined thresholds. This rate was chosen to minimize the mechanical irritation which could occur at higher flow rates particularly for the ocular mucosa. The olfactometer presented either clean air or the ammonia stock concentration (1000 ppm) diluted with clean air into 20 dilution steps (nominally from 500–2 ppm) through a Teflon nasal cannula, or a specially fitted set of goggles. The theoretical dilutions were confirmed to deviate slightly from the actual concentrations delivered with small losses in concentration likely due to adsorption phenomena within the delivery system. This required measurement of each stimulus concentration at the output of the olfactometer using a handheld photoionization detector. This was performed before and after each test session to determine the exact concentrations delivered to the nose/eyes for accurate calculations of the thresholds. A computer controlled the delivery duration and the side of presentation (left or right).
Testing
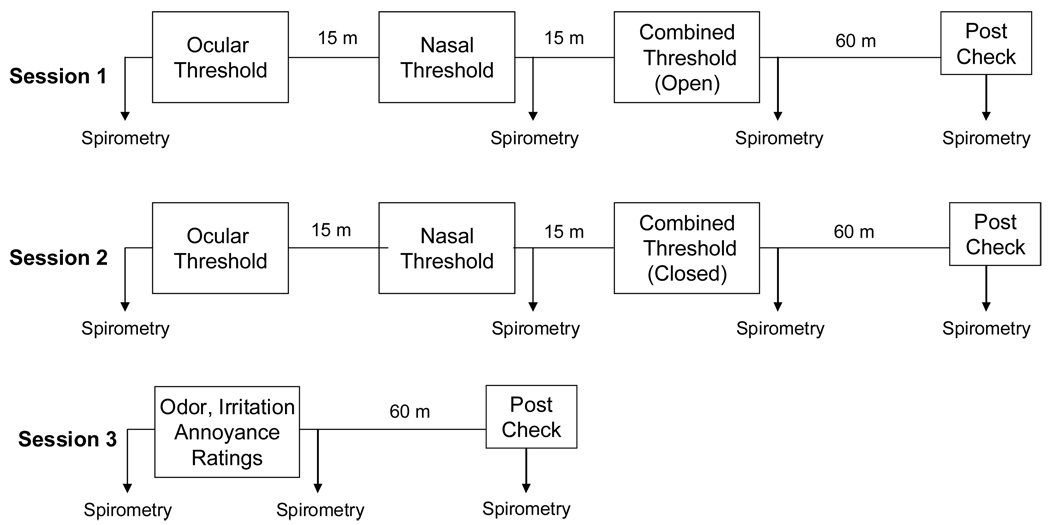
Figure 1 depicts the order of tests within each session.
Figure 1.
Experimental design: order of sessions and evaluations.
Irritation (Lateralization) Thresholds
The lateralization method to determine the sensory irritation threshold relies on the fact that the trigeminal system innervating the eyes and nasal mucosa can provide spatial localization information, whereas the olfactory system cannot. Unlike a simple detection task, the stimulus and clean air are simultaneously presented to the two nostrils or eyes and the participant is required to identify which nostril/eye had been stimulated, not just whether the stimulus has been detected. Thus when a volatile stimulus is delivered to one eye/nostril and clean air is delivered to the contralateral eye/nostril, an individual can reliably identify the stimulated side only when the threshold for trigeminal stimulation is reached or exceeded (Kobal et al. 1989;Kobal and Hummel 1990). The lateralization method used to obtain a threshold for trigeminal sensitivity using a two-alternative, forced-choice, up-down staircase procedure and calculating the threshold based on the mean of the last four reversals, described more fully in (Dalton et al. 2000).
In the current study, ammonia vapor flowed to either side of a set of specially-configured goggles (in the case of ocular exposure), to either nostril via a nasal cannula, or in the combination condition, to one eye and the ipsilateral nostril simultaneously. For nasal delivery, we adapted disposable low-flow nasal oxygen cannulas (Salter Labs, Arvin, CA) to accept the dual output of the olfactometer (either stimulus or clean air). The flexible prongs fitted loosely inside the nares of each subject, allowing them to receive the flow of vapor while slowly sniffing and exhaling. The goggles were configured by adapting a pair of watertight diving goggles (MFR, City) with isolated compartments for each eye. Each side of the goggles were drilled at the top to permit the insertion of a 1/16th “ Teflon tube which delivered the stimulus. An additional tube at 1 the bottom of the goggles was inserted to allow for vapor exhaust into a filtered exhaust system. The goggles fit snugly against the wearer’s face and thus provided isolation of the side receiving the ammonia vapor from the side receiving only the clean air on each trial. Four types of irritation thresholds were obtained (ocular, nasal and combined nasal/ocular with and without velopharyngeal closure). The velopharyngeal closure manipulation serves to isolate the throat and lower respiratory tract from the nasal passages and is a standard technique used in olfactometry studies (Dalton et al. 2006;Kobal and Hummel 1990;Nagel 1904). In this case, we utilized the technique to evaluate whether irritant receptors in the laryngeal area might play a role in heightened sensitivity to irritants, especially among asthmatics. This was done by instructing volunteers to raise their soft palate to restrict the vapor delivery to the upper airway alone and giving them practice with feedback prior to each experimental session. Failure to perform the maneuver would necessitate exclusion from the study, but all participants were able to perform the manipulation after a short practice session.
During ocular exposures, subjects were instructed to place their face into the goggle interface and close their eyes. After the olfactometer had delivered stimulus to one side of the goggles for 20 seconds and clean air to the other, the subject was instructed to open their eyes for a 10 second exposure per concentration. This was done to ensure the concentration of ammonia in the stimulated side of the goggle had reached equilibrium prior to exposure. They were then prompted to indicate which eye they believed received the stimulus. Nasal only exposures lasted for 10 seconds. Although the vapor delivery was largely passive, the 10 second delivery interval necessitated that the subject inhaled through their nose twice during this period, cued by the experimenter.. Subjects were then cued to exhale through both nostrils, rather than through the mouth. . At the end of each trial, they were prompted to indicate which nostril they believed received the stimulus. On each trial, stimulus presentation was randomized between left and right eyes or nostrils. The starting concentration for the single nasal and ocular irritation thresholds in each session was the fifth dilution step (~300 ppm).
Combined ocular/nasal thresholds were obtained by splitting the ammonia stream and delivering each half to one eye and the ipsilateral nares while clean air was presented to the contralateral side. The starting concentration for the combined ocular/nasal presentation trials was approximately two dilution steps less concentrated than that of the lower of the single ocular and nasal irritation thresholds obtained for that participant. The concentration was then varied in a step-wise fashion in order to determine the concentration at which the individual could reliably identify which eye/nostril was stimulated.
Intensity Ratings
On the third and final session, ratings of odor intensity, irritation and annoyance for ammonia vapor presented to one eye and nostril simultaneously were obtained. For this purpose, three concentrations were selected for each participant, based on their individual, previously determined combined (ocular/nasal) thresholds (two dilution steps above the threshold, at the threshold and two dilution steps below the threshold). Each concentration was presented three times in a randomized order.
After each stimulus concentration was flowed into the ipsilateral eye/nostril for 30 seconds, participants were asked to rate the overall odor intensity, annoyance, and perceived irritation (i.e., sensations of tingling, burning, stinging, warmth or coolness) experienced by moving a cursor to the appropriate position on a computerized line scale validated for between-group comparisons known as the general Labeled Magnitude Scale (gLMS) (Bartoshuk et al. 2004)). The gLMS categories ranged from ‘No Sensation’ to ‘Strongest Imaginable Sensation’ and the participants were instructed to compare their perception of a sensation (e.g. odor intensity) to all sensory experiences. Participants were also asked to indicate the site (ocular or nasal) at which they experienced the highest magnitude of irritation and/or annoyance.
Spirometry Measurements
Pulmonary function for all asthmatics and 15 healthy controls (a subset of the total control population) was evaluated at multiple timepoints throughout the testing sessions: at baseline (before the chemical exposure) and after the nasal and combined threshold measures. In addition, all asthmatic participants and their matched controls were required to remain at the Center for an additional hour following the last test whereupon an additional spirometric reading was obtained. The selection of the subset of healthy controls for this portion of the study was based on age and gender-matching to the asthmatic population.
For the spirometric measures, the “Snowbird Criteria” (Gardner 1988) was employed requiring at least three measurements of forced vital capacity (FVC) to be within 5% of each other (regarding shape of the curve and onset of expiration) without coughing, an unsatisfactory start to expiration, valsava maneuver, early termination of 1 expiration, or an unobstructed mouthpiece. For initial participant evaluation, the better value (i.e., the largest FVC and FEV1) of at least two reproducible measurements (within 5%) of FVC and FEV1 was used. Subjects who, after five attempts, could not provide two reproducible measurements were not required to provide any additional measurements, and the best value (i.e., the largest FVC and FEV1) obtained was used.
Statistical Analyses
Repeated-measure, analyses of variance (Statistica 6.0 ™) were used to evaluate differences in the ocular, nasal and combined thresholds within and between the asthmatics and healthy controls. Analysis of variance was also performed to evaluate differences in the ratings of odor, irritation and annoyance to the ocular/nasal presentation of ammonia vapor between these two groups. Finally, we subjected the FEV1 values to an analysis of variance to determine the significance of any changes in lung function among either group. Unless otherwise specified, significance was set at 0.05 for all tests.
Results
Lateralization Thresholds
Nasal and ocular thresholds collected on two separate 20 sessions did not differ across replicates: t (39) = 1.1, p=.27 (mean & SD =162 +/−29 vs. 21 189 +/− 32 ppm) and t (39) =1.2, p=.28 (mean & SD= 121 +/− 24 vs. 137 +/− 26 ppm) for nasal and ocular detection respectively. Accordingly, the means of the two nasal and ocular thresholds were used in all subsequent analyses. Table 2 depicts the mean ammonia thresholds in parts per million for each type of irritation threshold for both groups. There was no main effect of group: asthmatics and healthy controls did not differ in the threshold concentration necessary for lateralizing ammonia vapor in either the ocular, nasal or combined mucosa conditions.
Table 2.
Lateralization thresholds for ammonia detection for asthmatics and healthy controls in ppm ammonia.
| Ocular | Nasal | Combined (O) | Combined (Cl) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| Asthmatic | 133 | 25 | 167 | 31 | 94 | 22 | 77 | 12 |
| Healthy | 127 | 22 | 179 | 24 | 87 | 20 | 102 | 14 |
Across both groups, there was a main effect of threshold type, F (3, 117) = 10.45, p=.0001. Post-hoc analyses revealed that the ocular thresholds differed significantly from the nasal thresholds (Tukey’s HSD, p <.05) with the ocular thresholds being lower than the nasal thresholds (129 vs. 175 ppm). In addition, both of the combined thresholds (ocular/nasal) were significantly lower than either the individual nasal or ocular thresholds (Tukey’s HSD, p = .0001; 89 & 92 ppm vs. 129 and 175 ppm), suggesting some degree of spatial summation across sites of trigeminal stimulation.
Suprathreshold Ratings
Table 3 depicts the averaged ratings of odor, annoyance and irritation intensity for all three concentrations (below, at and above the irritation threshold), which were selected based on an individual’s combined threshold in the prior two sessions. A 2 (Group) × 3 (Concentration) × 3 (Rating type) ANOVA was performed and found a main effect of concentration, F (2, 4) = 3.75, p = .05, but neither a main effect of group F (1, 4) = .18, p 19 = .67, or rating F (2.,4) = .193, p = .825. Asthmatic participants did not perceive any of the concentrations to be more odorous, irritating or annoying than did the non-asthmatic participants. Both groups rated the odor intensity, annoyance, and perceived irritation (i.e., sensations of tingling, burning, stinging, warmth or coolness) between weak (at and below threshold) and moderate (above threshold) on the gLMS scale. The reports of and weak irritation at the concentrations individually determined to be at or below the irritation threshold would seem to require explanation. Given that subjects experienced longer stimulus durations in this part of the study (30 vs. 10 sec in threshold delivery) , one possibility is that the growth of irritation intensity is a function of Haber’s Law (Shusterman et al. 2006), where both concentration and duration determine stimulus 6 potency. As one illustration of this, over shorter durations (100–4000 ms), Wise (Wise et al. 2005) found that the irritancy from ammonia vapor exhibited some degree of temporal integration, such that rated irritation for a given concentration of ammonia increased with stimulus duration, Alternatively, or additionally, despite instructions to rate irritation based on the physical sensations in the nasal or ocular mucosa, it is not uncommon to observe ratings of perceived irritation occurring to concentrations which are in fact not a true irritant. This result often stems from confusion or bias when attempting to separately rate these two sensations in the nose, particularly when the odor quality is deemed unpleasant, and is in fact, why objective methods for irritation thresholds were developed (Cometto-Muñiz and Cain 1998;Wysocki et al. 2003). Significantly, however, there was no evidence that asthmatics tended to exhibit either greater irritancy at longer durations nor more bias to report irritation than did the healthy controls.
Table 3.
Average ratings of odor intensity, irritation and annoyance to three individually-determined concentrations of ammonia vapor.
| Asthmatic | Healthy | |||
|---|---|---|---|---|
| Mean | SEM | Mean | SEM | |
| Intensity BT | 8.6 | 2.23 | 8.27 | 1.62 |
| Intensity T | 9.5 | 1.66 | 9.25 | 1.44 |
| Intensity AT | 12.5 | 2.17 | 11.9 | 1.42 |
| Irritation BT | 6.9 | 1.51 | 8.7 | 1.32 |
| Irritation T | 10.6 | 1.39 | 9.7 | 1.72 |
| Irritation AT | 15.2 | 1.86 | 12.35 | 2.55 |
| Annoyance BT | 8.8 | 2.3 | 9 | 1.84 |
| Annoyance T | 8.9 | 1.6 | 9.57 | 2.12 |
| Annoyance AT | 13.5 | 2.09 | 11.03 | 2.3 |
BT = Below threshold; T = At threshold; AT = Above threshold
Spirometry Results
Decreases in FEV1 that fall between 0–5% of baseline are considered to be clinically insignificant and most likely represent changes induced by the effortful procedure itself. (Pellegrino et al. 2005). All but one of the spirometric readings were considered valid according to the ‘Snowbird Criteria’. Table 4 depicts the mean percentage change from baseline in FEV1 for each evaluation, averaged across all three sessions. There were no significant differences in FEV1 at any time points when compared to baseline, F (2,4) = 3 2.060, p = .163. Moreover, examination of the individual data found that no decreases greater than 5% in FEV1 were observed in any of the asthmatic volunteers or the subset of healthy controls at any time.
Table 4.
Average percentage change from baseline in forced expiratory volume (FEV1) following nasal and nasal/ocular exposure to ammonia for asthmatics and healthy controls.
| Post Nasal | Post Combined | Post 1 Hour | ||||
|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | |
| Asthmatic | 0.31 | −1.59 | −0.21 | −1.43 | 0.52 | −1.76 |
| Healthy | 0.26 | 1.03 | −0.11 | 0.92 | 0.31 | 1.03 |
Discussion and Conclusions
The goal of the current study was to compare mucosal sensitivity and bronchial reactivity between asthmatics and non-asthmatics when exposed to ammonia vapor. Prior studies evaluating nasal irritant sensitivity among individuals with pre-existing nasal inflammation (i.e. allergic or non-allergic rhinitis) found heightened irritant sensitivity in this group when compared to controls. Moreover, the existence of a naso-bronchial reflex suggests that stimulation of trigeminal receptors in the nasal mucosa could elicit or trigger broncho-constriction, a sequelae which would likely be more pronounced among asthmatics with hyperreactive airways. In contrast to the studies reporting elevations in nasal irritant sensitivity among individuals with allergic rhinitis and pre-existing nasal inflammation (Shusterman et al. 2003a;Shusterman et al. 2005), we did not observe heightened sensitivity among the asthmatic cohort evaluated here. On the surface, this may appear surprising given that nearly all asthmatic subjects reported a history of seasonal or perennial allergic rhinitis. However, while all may have experienced some degree of chronic rhinitis, none were exhibiting acute inflammatory or rhinitis symptoms at any of the test sessions, nor did they report experiencing acute nasal symptoms at any time between their test sessions. A recent study evaluating trigeminal sensitivity to electrical and thermal stimulation in the infraorbital, supraorbital, and mental dermatomes found a marked hypersensitivity to electrical stimulation among individuals who were experiencing acute sinusitis, but significant hyposensitivity to both stimuli among those with chronic sinusitis (Benoliel et al. 2006), raising the possibility that chemical stimulation of the trigeminal nerve may behave similarly. If so, then differential sensitivity to a volatile irritant may occur in the early versus later phases of inflammatory disease.
Despite the fact that asthmatics were exposed to concentrations of ammonia vapor that were at and above the irritant threshold, there was no evidence of a naso-bronchial reflex inducing changes in lung function. Perhaps more importantly, none of the asthmatics reported any increase in symptoms (nasal or pulmonary) during or following the experimental sessions. However, it is important to note that the maximum duration of exposure to supra-threshold concentrations in any session was less than five minutes and our results could underestimate the adverse effects exhibited from exposure to longer durations.
From a methodological standpoint, it is noteworthy that splitting the flow and delivering the ammonia vapor simultaneously to the nose and eyes, which is perhaps the most realistic exposure scenario other than a full-body exposure, did yield threshold estimates that were significantly lower than those obtained with either the eyes or nose alone. However, no significant differences were observed when comparing the two combined exposure conditions (laryngeal exposure or not), suggesting that the laryngeal region is not a significant target for irritation from ammonia. Physico-chemical factors may account for this result. Given the high water solubility of ammonia (425 g/L at 25 °C), it is likely that the ammonia vapor partitioned primarily into the anterior mucosal surfaces of the upper airways and thus little concentration remained in the air stream that eventually reached the laryngeal region (Zhao et al. 2004).
Although chamber studies utilizing full-body exposures are the gold standard for determining the degree to which real-world exposures can elicit symptoms or adverse effects, they are both difficult and expensive to conduct. In addition, chamber studies typically rely on self-reports or ratings of the onset of irritation, a procedure which can always be confounded by the concomitant presence of strong odor (Dalton 2003). While the present method of determining an irritant threshold does not completely mimic all aspects of potential real-world exposures to ammonia, the combined ocular and nasal exposure coupled with the objectivity of the forced-choice method does allow us to establish a concentration at or below which it is unlikely that any experiences of sensory irritation will occur at least over short exposure durations.
The question remains why we did not observe either greater sensitivity or reactivity among the asthmatic subjects in this study, given the widespread reports of adverse sensations and symptoms following exposure to many consumer products containing irritants or fragrances and other studies reporting asthmatic responses to fragrances or other volatile irritants (Elberling et al. 2005;Kumar et al. 1995;Shim and Williams 1986). Apart from the limitations related to the duration of exposure in this study, one possibility is that the cohort of asthmatic volunteers willing to participate in a challenge study such as the present one were not those most likely to exhibit symptoms from irritant exposures, based on their past experiences. However, prior to being tested, all asthmatic subjects we recruited did report some level of adverse response to household cleaning products, ranging from usually being bothered to always being bothered. Another possibility is that asthmatics responses in real-world situations can be modulated by concerns about the adverse effects they might experience from exposure to various agents. Although many volatile compounds can elicit irritation and trigger airways reflexes, reports of upper airway irritation and symptoms can also be elicited by non-chemical factors. For example, studies of healthy individuals have shown that expectancies related to the type of chemical or the exposure situation, modulated by factors such as exposure history or personality type, can lead to significant variation in an individual’s response to a chemical exposure. In several studies, characterizing an odorant as ‘industrial solvent’ elevated reports of irritation and other health symptoms significantly when compared to when the same odorant was characterized as a ‘natural aroma’ (Dalton 1996;Dalton 1999). The impact of these psychological factors was minimized in the present study, but is likely to be enhanced in everyday life and consequently have a greater potential for symptom exacerbation among individuals with pre-existing susceptibilities, such as asthma.
In conclusion, the ocular and nasal trigeminal system of mild-moderate asthmatics does not appear to be more sensitive or more reactive than that of non-asthmatics, nor does short duration exposure to ammonia at irritant levels induce changes in lung function. At least in brief exposures, the basis for some asthmatics to experience adverse responses to volatile compounds in everyday life may arise from factors other than trigeminally-mediated reflexes.
Acknowledgments
The authors wish to thank Laura Sitvarin and Christopher Schutz for assistance in testing participants and analytical procedures, and Carol Pierce, RN for assistance during the asthmatic portion of the study. We also thank Dr. Edward Emmett for his service as medical monitor for this study.
The research was supported in part by a grant from the SC Johnson Company and an NIH-NIDCD grant, RO1 DC 0488 to Pamela Dalton.
Reference List
- Bartoshuk LM, Duffy VB, Green BG, Hoffman HJ, Ko C-W, Lucchina LA, Marks LE, Snyder DJ, Weiffenbach JM. Valid across-group comparisons with labeled scales: the gLMS versus magnitude matching. Physiol Behav. 2004;82:109–114. doi: 10.1016/j.physbeh.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Benoliel R, Biron A, Quek SY, Nahlieli O, Eliav E. Trigeminal neurosensory changes following acute and chronic paranasal sinusitis. Quintessence Int. 2006;37:437–443. [PubMed] [Google Scholar]
- Cometto-Muñiz JE, Cain WS. Trigeminal and olfactory sensitivity: comparison of modalities and methods of measurement. International Archives of Occupational and Environmental Health. 1998;71:105–110. doi: 10.1007/s004200050256. [DOI] [PubMed] [Google Scholar]
- Dalton P. Odor perception and beliefs about risk. Chem Senses. 1996;21:447–458. doi: 10.1093/chemse/21.4.447. [DOI] [PubMed] [Google Scholar]
- Dalton P. Upper airway irritation, odor perception and health risk due to airborne chemicals. Toxicol Lett. 2003;140:239–248. doi: 10.1016/s0378-4274(02)00510-6. [DOI] [PubMed] [Google Scholar]
- Dalton P. Cognitive influences on health symptoms from acute chemical exposure. Health Psychol. 1999;18:579–590. doi: 10.1037//0278-6133.18.6.579. [DOI] [PubMed] [Google Scholar]
- Dalton P, Dilks D, Hummel T. Effect of long-term exposure to volatile irritants on sensory thresholds, negative mucosal potentials and event-related potentials. Behav Neurosci. 2006;120:180–187. doi: 10.1037/0735-7044.120.1.180. [DOI] [PubMed] [Google Scholar]
- Dalton P, Dilks DD, Banton MI. Evaluation of odor and sensory irritation thresholds for Methyl iso-Butyl Ketone (MiBK) in humans. Am Ind Hyg Assoc J. 2000;61:340–350. doi: 10.1080/15298660008984542. [DOI] [PubMed] [Google Scholar]
- Dalton P, Gould M, Girten B, Stodieck LS, Bateman TA. Preventing annoyance from odors in spaceflight: a method for evaluating the sensory impact of rodent housing. J Appl Physiol. 2003;95:2113–2121. doi: 10.1152/japplphysiol.00399.2003. [DOI] [PubMed] [Google Scholar]
- Elberling J, Linneberg A, Dirksen A, Johansen JD, Frolunds L, Madsen F, Nielsen NH. Mucosal symptoms elicited by fragrance products in a population-based sample in relation to atopy and bronchial hyper-reactivity. Clin Exp Allergy. 2005;35:75–81. doi: 10.1111/j.1365-2222.2005.02138.x. [DOI] [PubMed] [Google Scholar]
- Gardner RM. Standardization of spirometry: a summary of recommendations from the American Thoracic Society. The 1987 update. Ann Intern Med. 1988;108:217–220. doi: 10.7326/0003-4819-108-2-217. [DOI] [PubMed] [Google Scholar]
- Johansson A, Bende M, Millqvist E, Bake B. Nasobronchial relationship after cold air provocation. Respir Med. 2000;94:1119–1122. doi: 10.1053/rmed.2000.0924. [DOI] [PubMed] [Google Scholar]
- Kobal G, Hummel T. Brain responses to chemical stimulation of the trigeminal nerve in man. In: Green B, Mason JR, Kare MR, editors. Chemical Senses, vol 2: Irritation. New York: Marcel Dekken; 1990. pp. 123–136. [Google Scholar]
- Kobal G, Van Toller S, Hummel T. Is there directional smelling? Experientia. 1989;45:130–132. doi: 10.1007/BF01954845. [DOI] [PubMed] [Google Scholar]
- Kumar P, Caradonna-Graham VM, Gupta S, Cai X, Rao PN, Thompson J. Inhalation challenge effects of perfume scent strips in patients with asthma. Annals of Allergy, Asthma & Immunology. 1995;75:429–433. [PubMed] [Google Scholar]
- Nagel WA. Einige Bemerkungen über nasales Schmecken. Zeitschrift für Psychologie. 1904;25:268. [Google Scholar]
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- Plevkova J, Brozmanova M, Pecova R, Tatar M. Effects of intranasal capsaicin challenge on cough reflex in healthy human volunteers. J Physiol Pharmacol. 2004;55 Suppl 3:101–106. 101–106. [PubMed] [Google Scholar]
- Shim C, Williams HJ., Jr Effect of odors in asthma. Am J Med. 1986;80:18–22. doi: 10.1016/0002-9343(86)90043-4. [DOI] [PubMed] [Google Scholar]
- Shusterman D. Trigeminally-mediated health effects of air pollutants: sources of inter-individual variability. Hum Exp Toxicol. 2007;26:149–157. doi: 10.1177/0960327107070550. [DOI] [PubMed] [Google Scholar]
- Shusterman D, Matovinovic E, Salmon A. Does Haber's law apply to human sensory irritation? Inhal Toxicol. 2006;18:457–471. doi: 10.1080/08958370600602322. [DOI] [PubMed] [Google Scholar]
- Shusterman D, Murphy MA, Balmes J. Differences in nasal irritant sensitivity by age, gender, and allergic rhinitis status. Int Arch Occup Environ Health. 2003a;76:577–583. doi: 10.1007/s00420-003-0459-0. [DOI] [PubMed] [Google Scholar]
- Shusterman D, Murphy MA, Balmes J. Influence of age, gender, and allergy status on nasal reactivity to inhaled chlorine. Inhal Toxicol. 2003b;15:1179–1189. doi: 10.1080/08958370390229852. [DOI] [PubMed] [Google Scholar]
- Shusterman D, Tarun A, Murphy MA, Morris J. Seasonal allergic rhinitic and normal subjects respond differentially to nasal provocation with acetic acid vapor. Inhal Toxicol. 2005;17:147–152. doi: 10.1080/08958370590904508. [DOI] [PubMed] [Google Scholar]
- Smeets MA, Maute CM, Dalton P. Acute sensory irritation from exposure to isopropanol in workers and controls: Objective versus subjective effects. Ann Occup Hyg. 2002:359–373. doi: 10.1093/annhyg/mef054. [DOI] [PubMed] [Google Scholar]
- Togias A. Functional relationships between allergic rhinitis and asthma. Clinical and Experimental Allergy Reviews. 2003;3:18–22. [Google Scholar]
- Widdicombe JG. Reflexes from the Upper Respiratory Tract. In: Cherniak, Widdicombe JG, editors. Handbook of Physiology. American Physiological Society; 1986. pp. 363–394. [Google Scholar]
- Wise PM, Canty TM, Wysocki CJ. Temporal integration of nasal irritation from ammonia at threshold and supra-threshold levels. Toxicol Sci. 2005;87:223–231. doi: 10.1093/toxsci/kfi229. [DOI] [PubMed] [Google Scholar]
- Wysocki CJ, Cowart BJ, Radil T. Nasal trigeminal chemosensitivity across the adult life span. Percep Psychophys. 2003;65:115–122. doi: 10.3758/bf03194788. [DOI] [PubMed] [Google Scholar]
- Zhao K, Scherer PW, Hajiloo SA, Dalton P. Effect of anatomy on human nasal air flow and odorant transport patterns: Implications for olfaction. Chem Senses. 2004;29:365–379. doi: 10.1093/chemse/bjh033. [DOI] [PubMed] [Google Scholar]