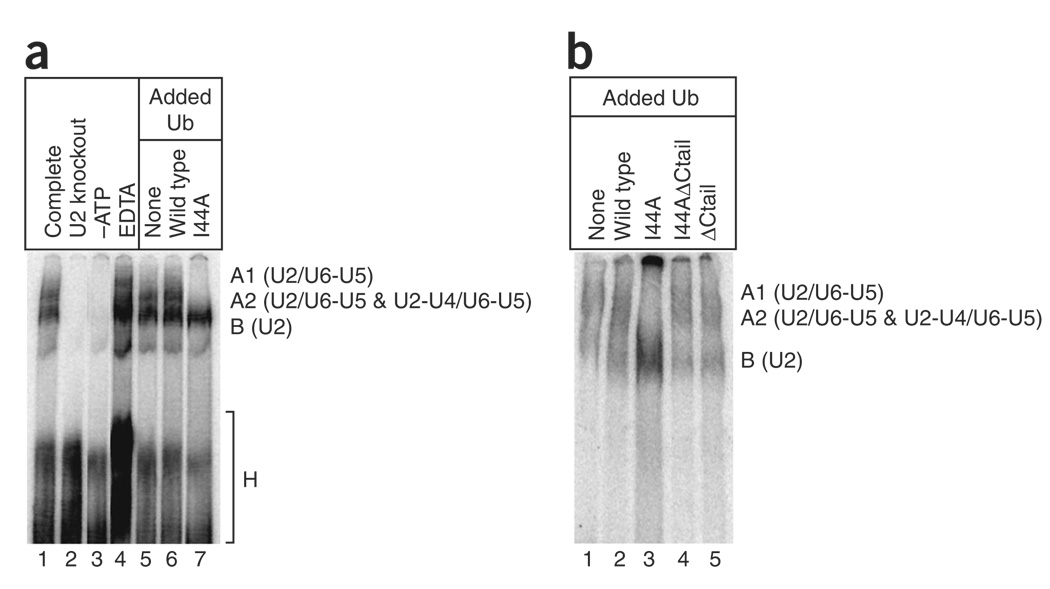
Figure 2.
The U2 snRNP-containing pre-spliceosome accumulates in the presence of I44A ubiquitin. (a) Radiolabeled pre-mRNA was incubated in splicing extract for 30 min, and the assembled complexes were then analyzed by native gel electrophoresis. The mobilities of H (nonspecific), B (U2), A2 (U2-U4/U6-U5 and U2/U6-U5), and A1 (U2/U6-U5) complexes are indicated on the right. The kinetically distinct A2-1 and A2-2 complexes47 were not resolved in this experiment. Lanes 1–4 were from control reactions that served to guide the identification of the complexes (lane 1, standard splicing reaction; lane 2, extract depleted of U2 snRNA by oligonucleotide-directed RNase H cleavage; lane 3, extract depleted of ATP by incubation with glucose; lane 4, extract supplemented with 5 mM EDTA, which leads to A1 complex accumulation47). Lanes 5–7 are from reactions with ubiquitin-depleted extract supplemented with buffer (lane 5), 1 mM wild-type ubiquitin (lane 6) or 1 mM I44A ubiquitin (lane 7). (b) As in a, except that the complete, U2 knockout, –ATP and EDTA reactions were omitted, and reactions supplemented with I44A ΔCtail ubiquitin (lane 4) and ΔCtail ubiquitin (lane 5) were included.