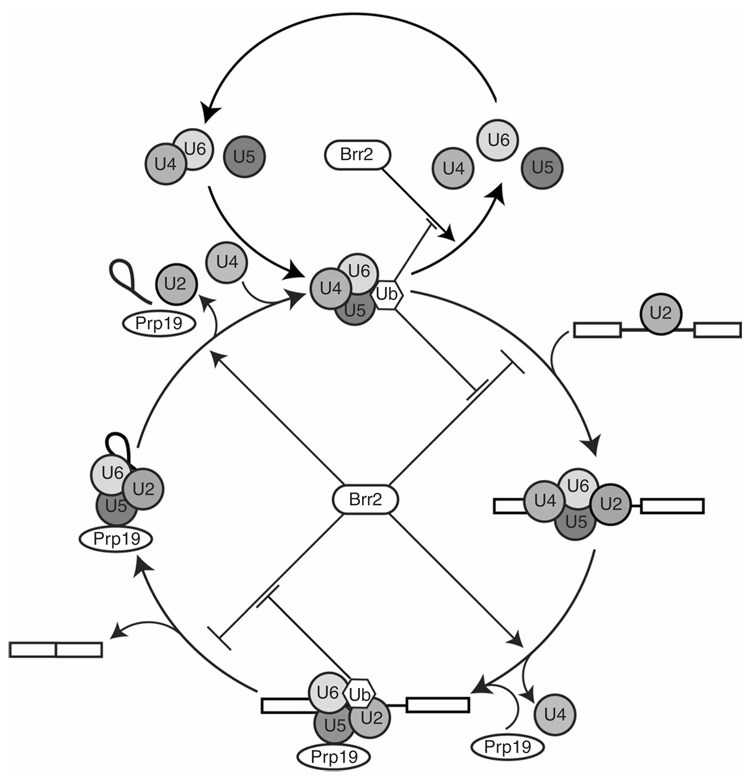
Figure 7.
A model for ubiquitin’s involvement in U4/U6-U5 triple snRNP accumulation and pre-mRNA splicing. The upper cycle depicts U4/U6-U5 triple snRNP assembly and disassembly23. The Brr2 ATPase promotes triple snRNP disassembly by catalyzing the unwinding of the U4 and U6 snRNAs4,23,35,36. Our results indicate that the recognition of a Prp8-ubiquitin conjugate within the U4/U6-U5 triple snRNP suppresses Brr2-catalyzed disassembly. The lower cycle depicts a hypothetical model for ubiquitin’s involvement in the complete pre-mRNA splicing pathway4,23,24. The U4/U6-U5 triple snRNP associates with the U2 snRNP-containing pre-spliceosome, and Brr2 can block this step by catalyzing triple snRNP disassembly; our results indicate that the recognition of a Prp8-ubiquitin conjugate suppresses Brr2 activity at this stage, allowing the triple snRNP to stably engage the pre-spliceosome. Once the spliceosome is assembled, the suppression of Brr2 activity is relieved (perhaps in part by the disruption of ubiquitin recognition), leading to U4/U6 unwinding and catalytic activation of the spliceosome. Upon spliceosome activation, Brr2 activity must once again be suppressed, to inhibit premature spliceosome disassembly probably due to Brr2-catalyzed U2/U6 unwinding4. We speculate that ubiquitin recognition is again involved in Brr2 inhibition at this stage. Once the splicing reaction is complete, the suppression of Brr2 activity is again overcome (perhaps in part by the disruption of ubiquitin recognition), leading to U2/U6 unwinding and spliceosome disassembly (ref. 4; E.C.S. and J.P.S., unpublished data). The points at which ubiquitin enters and exits the triple snRNP and spliceosome cycles are not known and are therefore omitted from the figure for clarity.