Abstract
We investigated the effects of anidulafungin alone and in combination with amphotericin B against Aspergillus fumigatus. Indifference was the only type of interaction observed in vitro. Anidulafungin at 1 and 5 mg/kg of body weight/day, amphotericin B at 1 mg/kg/day, and combination therapy prolonged the survival of mice with invasive aspergillosis. Anidulafungin at 5 mg/kg/day, alone and in combination with amphotericin B, reduced the kidney fungal burden. Overall, the combination was not superior to the most active single drug.
The high mortality rate of invasive aspergillosis has driven recent efforts to determine the efficacy of combination therapy in the treatment and management of those infections (1, 6, 7, 16, 17, 19, 20, 23, 29). Therefore, in this study, the in vitro and in vivo efficacies of the new echinocandin anidulafungin (AFG), alone and in combination with amphotericin B (AMB), against Aspergillus fumigatus were analyzed.
Three clinical strains (F2, F3, and F4) isolated from bronchoalveolar lavage specimens from patients with hematological diseases were identified to species level by conventional methods (24).
AMB was used as a pure powder (Sigma) for in vitro studies and as a commercial preparation (Fungizone; Bristol-Myers Squibb) for in vivo studies. Pure powder of AFG (Pfizer) was dissolved in dimethyl sulfoxide and further diluted in the test medium or sterile saline solution for in vitro and in vivo studies, respectively.
MICs and minimum effective concentrations (MECs; the lowest concentrations that led to the growth of small, rounded, compact hyphal forms compared to the hyphal growth seen in the growth control well) were determined in RPMI 1640 medium by the CLSI M38-A2 broth microdilution method (10, 12).
For both susceptibility and checkerboard assays, the MICs and MECs were read visually at 24 and 48 h (10, 25). Drug interactions were classified as synergistic, indifferent, or antagonistic based on the fractional inhibitory concentration (FIC) index (16).
Minimum fungicidal concentration (MFC) was considered the concentration of antifungal agents, alone or in combination, that yielded no growth (27).
Metabolic activities of conidia and hyphae were assessed in RPMI 1640 medium with l-glutamine, without phenol red and NaHCO3, by XTT [2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxyanilide inner salt] assay (Tox-2; Sigma) (2, 3, 21).
An experimental CD1 mouse (Charles River, Calco, Italy) model of invasive aspergillosis was used by following previously reported procedures (4). A total of three separate in vivo studies were performed by injection of the A. fumigatus F3 isolate. The drug treatments were started 2 h after the infection. AMB at 0.5 and 1 mg/kg of body weight/day, AFG at 1 and 5 mg/kg/day, and combination doses were administered intraperitoneally.
In survival studies, the mice were treated daily from day 0 to day 4 and observed for 10 consecutive days.
Brain and kidney fungal burdens were determined at day 4 postinfection by CFU count and quantitative PCR based on procedures described by Bowman et al. (5).
Histopathology analysis was performed at day 4 postinfection (4). The number of fungal microabscesses was evaluated in 20 consecutive microscopic fields. Each section was classified based on the number of fungal microabscesses as follows: absence, <5, ≥5 to ≤20, and >20.
The in vitro results were analyzed by either Mann-Whitney U test or Student's t test, with a P value of <0.05 considered significant. Survival and tissue burden studies were analyzed by log rank and Mann-Whitney U tests, respectively. Due to multiple comparisons, a P value of <0.016 was considered significant.
Our in vitro results are shown in Table 1. Overall, AFG MECs were significantly lower than AMB MICs and indifference was the only type of interaction among the two drugs.
TABLE 1.
In vitro susceptibility tests of AFG and AMB, alone and in combination, against three clinical isolates of A. fumigatusa
| Isolate | Drug(s) | MIC or MEC (μg/ml) at 24 h
|
MIC or MEC (μg/ml) at 48 h
|
MFC (μg/ml) at 48 h
|
|||
|---|---|---|---|---|---|---|---|
| Median | Range | Median | Range | Median | Range | ||
| F2 | AFG | 0.001 | 0.001-0.002 | 0.002 | 0.002 | >16 | >16 |
| AMB | 0.5 | 0.25-0.5 | 1.0 | 1.0 | 1.0 | 1.0-2.0 | |
| AFG + AMBb | 0.001/0.03 | 0.001-0.002/0.03-0.06 | 0.001/0.06 | 0.001-0.002/0.03-0.06 | <0.0002/1.0 | <0.0002/1.0-2.0 | |
| F3 | AFG | 0.001 | 0.001-0.002 | 0.002 | 0.002 | >16 | >16 |
| AMB | 0.25 | 0.25-0.5 | 1.0 | 0.5-1.0 | 1.0 | 0.5-1.0 | |
| AFG + AMBb | 0.001/0.03 | 0.001/0.03 | 0.002/0.03 | 0.002-0.03 | <0.0002/1.0 | <0.0002/0.5-1.0 | |
| F4 | AFG | 0.002 | 0.001-0.002 | 0.002 | 0.002 | >16 | >16 |
| AMB | 0.25 | 0.125-0.5 | 1.0 | 1.0-2.0 | 1.0 | 0.5-1.0 | |
| AFG + AMBb | 0.002/0.25 | 0.001-0.002/0.03-0.25 | 0.002/0.25 | 0.001-0.002/0.25 | <0.0002/1.0 | <0.0002/0.5-1.0 | |
Each test was run in triplicate and repeated on two different days.
The MEC values were reported as end point readings of the checkerboard assays. The interaction between the drugs was classified as “indifferent” in each case (interactions were defined as synergistic if the FIC index was less than or equal to 0.50, indifferent if the FIC index was greater than 0.50 and less than or equal to 4.0, and antagonistic if the FIC index was greater than 4.0).
MFC values for AFG were all >16 μg/ml, while the combination values were statistically lower than those for AFG alone, but not for AMB alone.
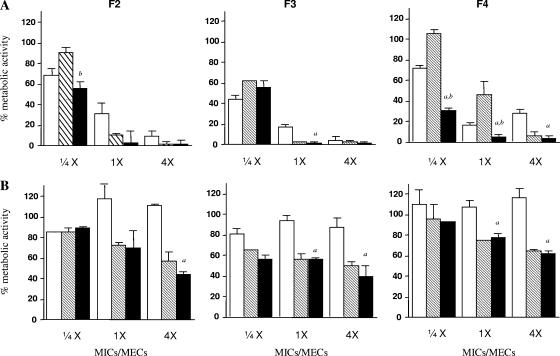
The studies of metabolic activity either on conidia or on hyphae are presented in Fig. 1.
FIG. 1.
Percentages of metabolic activity of three clinical isolates of A. fumigatus (F2, F3, and F4) at the stage of nongerminated conidia (A) and filamentous forms (B) detected by XTT assay. AFG (white bars), AMB (striped bars), and the combination of the two antifungal agents (black bars) were tested to concentrations of 1/4, 1, and 4 times the respective MICs and MECs. The bars represent the means of percent metabolic activity in the presence of the drugs with respect to the growth controls. The error bars indicate the standard deviations of the means. Letters a and b indicate reduced metabolic activity of the combination versus AFG and AMB alone, respectively (P < 0.05). Each strain was tested in triplicate.
AFG, AMB, and the combination regimens showed a dose-dependent reduction of metabolic activity against conidia, but generally the combination was not more effective than the most active drug alone. Against the hyphae, AMB showed a decreased activity, while AFG was not active. The combination was effective, but not more effective than AMB alone.
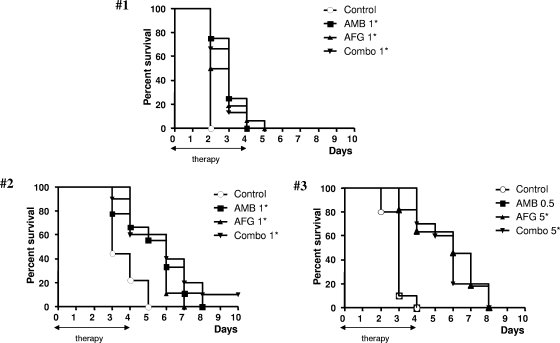
The in vivo results are shown in Fig. 2. In studies 1 and 2, all drug regimens prolonged significantly the survival over that of control animals. In both studies, the groups treated with the combination regimens did not have significantly increased survival times with the respect to the AMB- and AFG-treated groups. In study 3, AFG at 5 mg/kg/day and the combination regimen, but not AMB at 0.5 mg/kg/day, significantly prolonged the survival time with the respect to the control group. Combination treatment did not extend survival beyond that of the AFG-treated group.
FIG. 2.
Survival of mice infected intravenously with the A. fumigatus F3 clinical isolate. In study 1, the animals were infected with 1.5 × 107 conidia/mouse and treated with AMB at 1 mg/kg/day, AFG at 1 mg/kg/day, and the respective combination regimen; in study 2, the mice were infected with 3.5 × 105 conidia/mouse and treated with AMB at 1 mg/kg/day, AFG at 1 mg/kg/day, and the respective combination; in study 3, the animals were infected with 3.2 × 106 A. fumigatus conidia/mouse and treated with AMB at 0.5 mg/kg/day, AFG at 5 mg/kg/day, and the corresponding combination. The therapies were started 2 h postinfection (day 0) and continued through day 4 postinfection (five consecutive days). There were from 9 to 16 mice in each group. Asterisks indicate groups with prolonged survival over controls (due to multiple comparisons, P values of <0.016 were considered statistically significant).
Kidney and brain burden results are shown in Table 2. Only AFG at 5 mg/kg/day and the respective combination with AMB were effective at reducing the CFU or conidial equivalent per gram of kidney tissues. No treatments were effective at reducing the brain burdens.
TABLE 2.
Fungal burden in tissues of A. fumigatus-infected mice measured by CFU and quantitative PCR assaysa
| Challenge dose (conidia/mouse) | Treatment (drug, dose [mg/kg/day]) | Brain burden
|
Kidney burden
|
||
|---|---|---|---|---|---|
| Mean log10 CFU/g of tissue ± SD | Mean log10 CE/g of tissue ± SD | Mean log10 CFU/g of tissue ± SD | Mean log10 CE/g of tissue ± SD | ||
| 3.5 × 105 | Control | 3.17 ± 0.44 | 4.49 ± 0.35 | 5.12 ± 0.26 | 7.85 ± 0.57 |
| AMB, 1 | 2.51 ± 0.74 | 3.73 ± 0.81 | 4.74 ± 0.43 | 7.17 ± 0.64 | |
| AFG, 1 | 3.02 ± 0.83 | 4.50 ± 1.09 | 4.95 ± 0.27 | 7.42 ± 0.59 | |
| AMB, 1 + AFG, 1 | 3.06 ± 0.60 | 4.78 ± 0.47 | 4.89 ± 0.44 | 7.78 ± 0.53 | |
| 3.2 × 106 | Control | 3.73 ± 0.25 | 5.60 ± 0.36 | 5.31 ± 0.15 | 7.68 ± 0.22 |
| AMB, 0.5 | 3.82 ± 0.22 | 5.88 ± 0.71 | 4.96 ± 0.32 | 7.36 ± 0.34 | |
| AFG, 5 | 4.08 ± 0.40 | 6.27 ± 0.65 | 4.60 ± 0.50* | 6.61 ± 0.66* | |
| AMB, 0.5 + AFG, 5 | 4.16 ± 0.71 | 6.58 ± 0.98 | 4.24 ± 0.39* | 6.50 ± 0.62* | |
The animals were infected with the A. fumigatus F3 isolate (3.5 × 105 conidia/mouse and 3.2 × 106 conidia/mouse in studies 2 and 3, respectively) and euthanized 3 days later. There were seven animals per group, and fungal burdens of brains and kidneys were determined by measuring CFU or conidial equivalents (CE) per gram of tissue. Asterisks indicate treatment groups with reduced fungal burdens over the controls (due to multiple comparisons, P values of <0.016 were considered statistically significant).
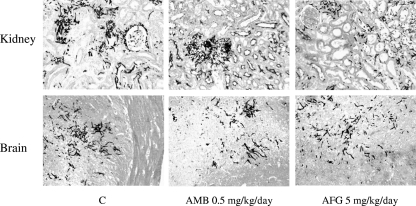
Consistent with these data, a decreased number of fungal microabscesses were observed in kidney tissues, but not in brain tissues, of mice treated with AFG at 5 mg/kg/day (Fig. 3).
FIG. 3.
Histopathological sections of kidney and brain tissues stained with Grocott Gomori (original magnification, ×25) from mice infected with 3.2 × 106 conidia of the A. fumigatus F3 isolate. Representative histopathological sections of kidney and brain tissues from control mice (C) and from mice treated for three consecutive days with AMB at 0.5 mg/kg/day and AFG at 5 mg/kg/day are shown.
Our AFG MEC values were similar to those previously reported for A. fumigatus isolates (12, 22). In agreement with a previous study conducted by Philip et al. (28), AFG used in combination with the polyene yielded an indifferent type of interaction.
Our in vivo results showed that AFG given at 1 and 5 mg/kg/day was effective at prolonging survival. These data correspond to those already reported for other experimental models of aspergillosis (26, 32).
Here, we found that the combination was not more effective than the most active drug alone in all three survival experiments.
In terms of kidney tissue burdens, we found that AFG given at 5 mg/kg/day, but not at 1 mg/kg/day, reduced fungal burden with respect to that in untreated controls. The combination was not more active than AFG alone. Several published studies have already explored the effects of echinocandins other than AFG combined with various AMB formulations against Aspergillus spp. Although two studies suggested that there were beneficial effects from combined therapies (i.e., caspofungin plus AMB and micafungin plus AMB) over the monotherapies (11, 30), most studies showed that combinations did not enhance the effects of the most active single drug (8, 9, 13, 15, 18, 31).
We showed that neither single drugs nor combinations were active in brain tissues. The lack of AFG efficacy in brains, but not in kidneys, might be explained by its pharmacokinetics features. Groll et al. (14) have studied the AFG tissue distribution in healthy rabbits and reported an undetectable cerebrospinal fluid concentration. Overall, our results showed that the new echinocandin AFG has the potential to be used as a therapeutic treatment against invasive aspergillosis. The combination therapy of AFG with AMB did not improve the outcomes analyzed in the present study, although antagonism was not observed.
Footnotes
Published ahead of print on 13 July 2009.
REFERENCES
- 1.Aliff, T. B., P. G. Maslak, J. G. Jurcic, M. L. Heaney, K. N. Cathcart, K. A. Sepkowitz, and M. A. Weiss. 2003. Refractory Aspergillus pneumonia in patients with acute leukemia: successful therapy with combination caspofungin and liposomal amphotericin. Cancer 97:1025-1032. [DOI] [PubMed] [Google Scholar]
- 2.Antachopoulos, C., J. Meletiadis, T. Sein, E. Roilides, and T. J. Walsh. 2007. Concentration-dependent effects of caspofungin on the metabolic activity of Aspergillus species. Antimicrob. Agents Chemother. 51:881-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antachopoulos, C., J. Meletiadis, T. Sein, E. Roilides, and T. J. Walsh. 2008. Comparative in vitro pharmacodynamics of caspofungin, micafungin, and anidulafungin against germinated and nongerminated Aspergillus conidia. Antimicrob. Agents Chemother. 52:321-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barchiesi, F., E. Spreghini, A. Santinelli, A. W. Fothergill, E. Pisa, D. Giannini, M. G. Rinaldi, and G. Scalise. 2007. Posaconazole prophylaxis in experimental systemic zygomycosis. Antimicrob. Agents Chemother. 51:73-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowman, J. C., G. K. Abruzzo, J. W. Anderson, A. M. Flattery, C. J. Gill, V. B. Pikounis, D. M. Schmatz, P. A. Liberator, and C. M. Douglas. 2001. Quantitative PCR assay to measure Aspergillus fumigatus burden in a murine model of disseminated aspergillosis: demonstration of efficacy of caspofungin acetate. Antimicrob. Agents Chemother. 45:3474-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caillot, D., A. Thiébaut, R. Herbrecht, S. de Botton, A. Pigneux, F. Bernard, J. Larché, F. Monchecourt, S. Alfandari, and L. Mahi. 2007. Liposomal amphotericin B in combination with caspofungin for invasive aspergillosis in patients with hematologic malignancies: a randomized pilot study (Combistrat trial). Cancer 110:2740-2746. [DOI] [PubMed] [Google Scholar]
- 7.Chamilos, G., and D. P. Kontoyiannis. 2006. The rationale of combination antifungal therapy in severely immunocompromised patients: empiricism versus evidence-based medicine. Curr. Opin. Infect. Dis. 19:380-385. [DOI] [PubMed] [Google Scholar]
- 8.Clemons, K. V., R. Parmar, M. Martinez, and D. A. Stevens. 2006. Efficacy of Abelcet alone, or in combination therapy, against experimental central nervous system aspergillosis. J. Antimicrob. Chemother. 58:466-469. [DOI] [PubMed] [Google Scholar]
- 9.Clemons, K. V., and D. A. Stevens. 2006. Efficacy of micafungin alone or in combination against experimental pulmonary aspergillosis. Med. Mycol. 44:69-73. [DOI] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard M38-A2, 2nd ed. Clinical and Laboratory Standards Institute, Wayne, PA.
- 11.Dennis, C. G., W. R. Greco, Y. Brun, R. Youn, H. K. Slocum, R. J. Bernacki, R. Lewis, N. Wiederhold, S. M. Holland, R. Petraitiene, T. J. Walsh, and B. H. Segal. 2006. Effect of amphotericin B and micafungin combination on survival, histopathology, and fungal burden in experimental aspergillosis in the p47phox−/− mouse model of chronic granulomatous disease. Antimicrob. Agents Chemother. 50:422-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espinel-Ingroff, A., A. Fothergill, M. Ghannoum, E. Manavathu, L. Ostrosky-Zeichner, M. A. Pfaller, M. G. Rinaldi, W. Schell, and T. J. Walsh. 2007. Quality control and reference guidelines for CLSI broth microdilution method (M38-A document) for susceptibility testing of anidulafungin against molds. J. Clin. Microbiol. 45:2180-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graybill, J. R., R. Bocanegra, G. M. Gonzalez, and L. K. Najvar. 2003. Combination antifungal therapy of murine aspergillosis: liposomal amphotericin B and micafungin. J. Antimicrob. Chemother. 52:656-662. [DOI] [PubMed] [Google Scholar]
- 14.Groll, A. H., D. Mickiene, R. Petraitiene, V. Petraitis, C. A. Lyman, J. S. Bacher, S. C. Piscitelli, and T. J. Walsh. 2001. Pharmacokinetic and pharmacodynamic modeling of anidulafungin (LY303366): reappraisal of its efficacy in neutropenic animal models of opportunistic mycoses using optimal plasma sampling. Antimicrob. Agents Chemother. 45:2845-2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imai, J., G. Singh, B. Fernandez, K. V. Clemons, and D. A. Stevens. 2005. Efficacy of Abelcet and caspofungin, alone or in combination, against CNS aspergillosis in a murine model. J. Antimicrob. Chemother. 56:166-171. [DOI] [PubMed] [Google Scholar]
- 16.Johnson, M. D., C. MacDougall, L. Ostrosky-Zeichner, J. R. Perfect, and J. H. Rex. 2004. Combination antifungal therapy. Antimicrob. Agents Chemother. 48:693-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kontoyiannis, D. P., R. Hachem, R. E. Lewis, G. A. Rivero, H. A. Torres, J. Thornby, R. Champlin, H. Kantarjian, G. P. Bodey, and I. I. Raad. 2003. Efficacy and toxicity of caspofungin in combination with liposomal amphotericin B as primary or salvage treatment of invasive aspergillosis in patients with hematologic malignancies. Cancer 98:292-299. [DOI] [PubMed] [Google Scholar]
- 18.Luque, J. C., K. V. Clemons, and D. A. Stevens. 2003. Efficacy of micafungin alone or in combination against systemic murine aspergillosis. Antimicrob. Agents Chemother. 47:1452-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maertens, J., A. Glasmacher, R. Herbrecht, A. Thiebaut, C. Cordonnier, B. H. Segal, J. Killar, A. Taylor, N. Kartsonis, T. F. Patterson, M. Aoun, D. Caillot, C. Sable, and the Caspofungin Combination Therapy Study Group. 2006. Multicenter, noncomparative study of caspofungin in combination with other antifungals as salvage therapy in adults with invasive aspergillosis. Cancer 107:2888-2897. [DOI] [PubMed] [Google Scholar]
- 20.Marr, K. A., M. Boeckh, R. A. Carter, H. W. Kim, and L. Corey. 2004. Combination antifungal therapy for invasive aspergillosis. Clin. Infect. Dis. 39:797-802. [DOI] [PubMed] [Google Scholar]
- 21.Meshulam, T., S. M. Levitz, L. Christin, and R. D. Diamond. 1995. A simplified new assay for assessment of fungal cell damage with the tetrazolium dye, (2,3)-bis-(2-methoxy-4-nitro-5-sulphenyl)-(2H)-tetrazolium-5-carboxanilide (XTT). J. Infect. Dis. 172:1153-1156. [DOI] [PubMed] [Google Scholar]
- 22.Messer, S. A., G. J. Moet, J. T. Kirby, and R. N. Jones. 2009. Activity of contemporary antifungal agents, including the novel echinocandin anidulafungin, tested against Candida spp., Cryptococcus spp., and Aspergillus spp.: report from the SENTRY antimicrobial surveillance program (2006 to 2007). J. Clin. Microbiol. 47:1942-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukherjee, P. K., D. J. Sheehan, C. A. Hitchcock, and M. A. Ghannoum. 2005. Combination treatment of invasive fungal infections. Clin. Microbiol. Rev. 18:163-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray, P. R., E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.). 2003. Manual of clinical microbiology, 8th ed. ASM Press, Washington, DC.
- 25.Odds, F. C., M. Motyl., R. Andrade, J. Bille, E. Canton, M. Cuenca-Estrella, A. Davidson, C. Durussel, D. Ellis, E. Foraker, A. W. Fothergill, M. A. Ghannoum, R. A. Giacobbe, M. Gobernado, R. Handke, M. Laverdiere, W. Lee-Yang, W. G. Merz, L. Ostrosky-Zeichner, J. Peman, S. Perea, J. R. Perfect, M. A. Pfaller, L. Proia, J. H. Rex, M. G. Rinaldi, J. L. Rodriguez-Tudela, W. A. Schell, C. Shields, D. A. Sutton, P. E. Verweij, and D. W. Warnock. 2004. Interlaboratory comparison of results of susceptibility testing with caspofungin against Candida and Aspergillus species. J. Clin. Microbiol. 42:3475-3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petraitis, V., R. Petraitiene, A. H. Groll, A. Bell, D. P. Callender, T. Sein, R. L. Schaufele, C. L. McMillian, J. Bacher, and T. J. Walsh. 1998. Antifungal efficacy, safety, and single-dose pharmacokinetics of LY303366, a novel echinocandin B, in experimental pulmonary aspergillosis in persistently neutropenic rabbits. Antimicrob. Agents Chemother. 42:2898-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfaller, M. A., D. J. Sheehan, and J. H. Rex. 2004. Determination of fungicidal activities against yeasts and molds: lessons learned from bactericidal testing and the need for standardization. Clin. Microbiol. Rev. 17:268-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Philip, A., Z. Odabasi, J. Rodriguez, V. L. Paetznick, E. Chen, J. H. Rex, and L. Ostrosky-Zeichner. 2005. In vitro synergy testing of anidulafungin with itraconazole, voriconazole, and amphotericin B against Aspergillus spp. and Fusarium spp. Antimicrob. Agents Chemother. 49:3572-3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Segal, B. H., and W. J. Steinbach. 2007. Combination antifungals: an update. Expert Rev. Anti Infect. Ther. 5:883-892. [DOI] [PubMed] [Google Scholar]
- 30.Sionov, E., S. Mendlovic, and E. Segal. 2006. Efficacy of amphotericin B or amphotericin B-intralipid in combination with caspofungin against experimental aspergillosis. J. Infect. 53:131-139. [DOI] [PubMed] [Google Scholar]
- 31.Sivak, O., K. Bartlett, V. Risovic, E. Choo, F. Marra, D. S. Batty, Jr., and K. M. Wasan. 2004. Assessing the antifungal activity and toxicity profile of amphotericin B lipid complex (ABLC; Abelcet) in combination with caspofungin in experimental systemic aspergillosis. J. Pharm. Sci. 93:1382-1389. [DOI] [PubMed] [Google Scholar]
- 32.Verweij, P. E., K. L. Oakley, J. Morrissey, G. Morrissey, and D. W. Denning. 1998. Efficacy of LY303366 against amphotericin B-susceptible and -resistant Aspergillus fumigatus in a murine model of invasive aspergillosis. Antimicrob. Agents Chemother. 42:873-878. [DOI] [PMC free article] [PubMed] [Google Scholar]