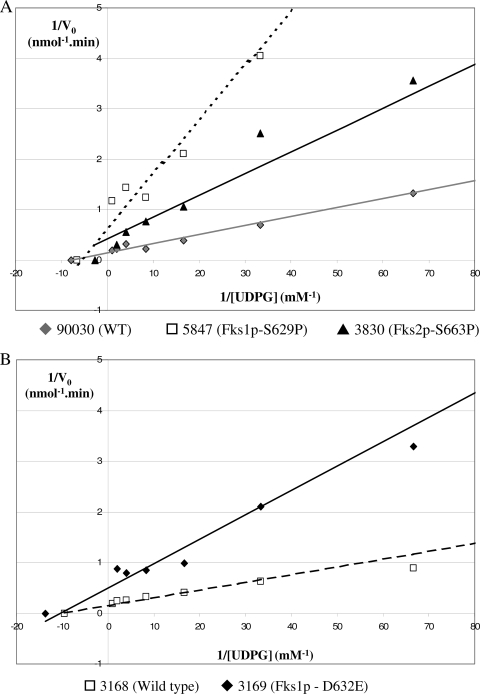
FIG. 3.
Lineweaver-Burke double-reciprocal plots used to determine the maximum velocity (Vmax) and the Michaelis-Menten constant (Km) for product-entrapped 1,3-β-d-glucan synthase complexes by varying the amount of [3H]UDPG. (A) Comparison of the kinetic properties of the 1,3-β-d-glucan synthase enzymes isolated from strains ATCC 90030 (wild type [WT]), 5847 (Fks1p-S629P), and 3830 (Fks2p-S663P). (B) Evaluation of the kinetic properties of the 1,3-β-d-glucan synthase enzyme obtained from the isogenic strains 3168 (WT) and 3169 (Fks1p-D632G).