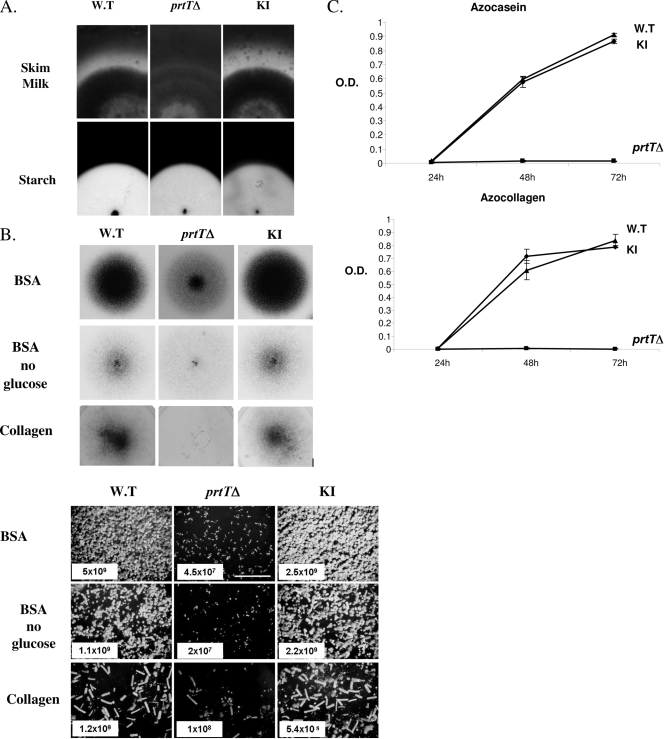
FIG. 3.
Characterization of PrtT-dependent proteolytic activity and growth. (A) The prtTΔ mutant does not form a proteolytic halo when grown on SM agar plates (top panels). Conidia from the AF293 WT, prtTΔ, and reconstituted (KI) strains were point inoculated on SM plates and grown for 48 h at 37°C and then for another 48 h at room temperature. The formation of a proteolytic halo was highlighted by Coomassie blue staining. Lower panels, all three strains form normal clearance zones on starch-containing agar plates, suggesting that amylase secretion is not impaired in the prtTΔ mutant. (B) Reduced conidiation and growth of the prtTΔ mutant on agar plates containing BSA or collagen as sole nitrogen and carbon sources. The top panels illustrate the gross colonial morphology of the strains point inoculated and grown for 48 h at 37°C. The lower panels show conidiophore density (magnification, ×40) and conidial counts (total number of conidia/plate) of the strains in spread culture. (C) Time course of azocasein and azocollagen degradation by the WT, prtTΔ, and KI strains. Strains were grown for 24, 48, and 72 h at 37°C in shaking SM liquid culture. Supernatants were incubated with azocasein or azocollagen, and the proteolytic release of azo dye was determined by measuring the absorbance at 436 or 520 nm, respectively. O.D., optical density. The results are representative of three independent experiments performed in triplicate and are expressed as the means ± standard deviations for three replicates.