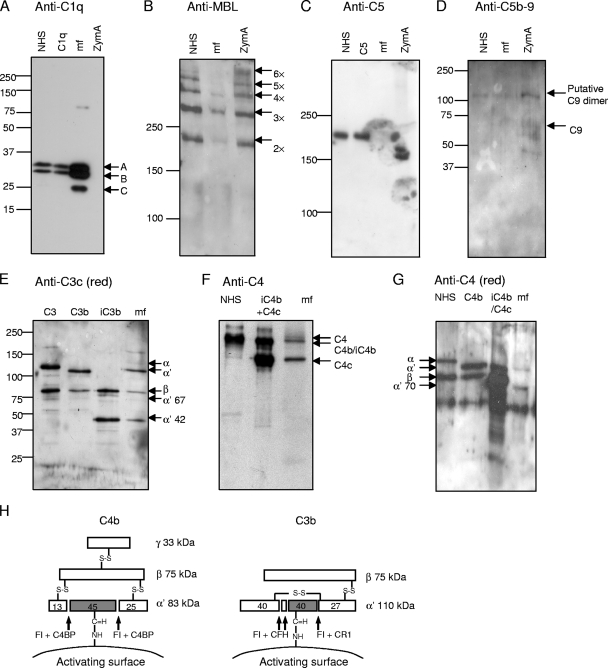
FIG. 1.
Deposition of complement components C1q, MBL, and C5; C5b-9 complexes; and fragments of C3 and C4 on the surface of Loa loa MF. The presence of those molecules on MF was analyzed after subjecting purified patient blood-derived MF to either acidic elution (A, B, and C) or elution with reducing buffer (G) or after homogenizing MF (D, E, and F). The samples obtained were used in SDS-polyacrylamide gel electrophoresis under either reducing (A, D, E, and G) or nonreducing (B, C, and F) conditions and subsequent Western blotting with antibodies against the molecules as indicated above each panel (the anti-C5b-9 panel was blotted with anti-C9 neoepitope antibody). As positive controls, complement components C1q (0.3 μg/ml); C5 (1 μg/ml); C3, C3b, and iC3b (0.02 μg each); C4b and iC4b (0.04 μg each); and NHS were used (diluted 1:500 for panel A, 1:100 for panel B, 1:200 for panel C, and 1:1,000 for panels F and G). NHS was used as a negative control for C5b-9 deposition (D). NHS-exposed zymosan A (ZymA) was used as a positive control in the deposition of the complement components MBL (B) and C5b-9 complex (D) and as a negative control in the deposition of C1q (A). The mobility of molecular size markers is indicated on the left sides. Mobilities of the C1q subunits A, B, and C (A); the oligomers of MBL (B); the activated C5b-9 associated C9 complexes (D); the fragments of C3 (E); and the fragments of C4 (F and G) are indicated. A schematic presentation of C4b and C3b fragments and the cleavage sites of FI is shown in panel H.