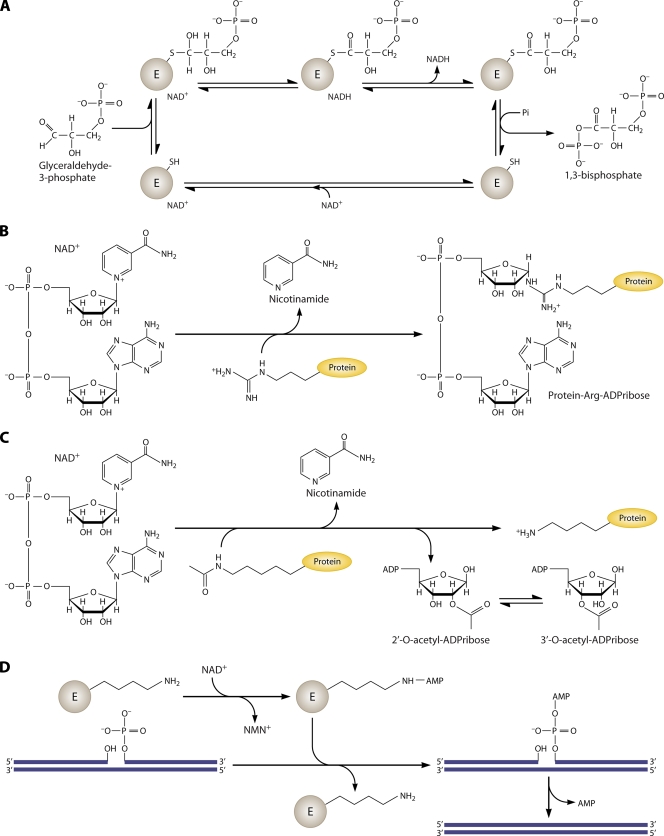
FIG. 1.
Biochemical reactions of NAD. (A) NAD is utilized as a coenzyme in the conversion of glyceraldehyde 3-phosphate to 1,3-bisphosphate by glyceraldehyde phosphate dehydrogenase. The enzyme-bound NAD promotes formation of a covalent thiohemiacetal intermediate and conversion to a dehydrogenated thioester with conversion of the coenzyme to NADH. The bound thioester is then phosphorylated to the 1,3-bisphosphate product. (B) NAD is the ADP-ribose donor for ADP-ribose transfer reactions by ADP-ribose transferases. The depicted reaction involves protein arginine ADP-ribosylation with production of nicotinamide. (C) Sirtuins are NAD-dependent protein lysine deacetylases. In sirtuin reactions, NAD is the acetyl acceptor, forming 2′- and 3′-acetylated ADP-ribose plus nicotinamide and the nonmodified protein lysine, from an acetylated protein Lys. (D) Bacterial DNA ligases utilize NAD to adenylylate the ligase active-site lysine residue, which activates the 5′ phosphate of a nicked DNA substrate, forming an adenylylated nicked substrate. The enzyme then promotes the attack of the 3′ hydroxyl on the 5′ phosphate, releasing AMP and forming a DNA phosphodiester bond.