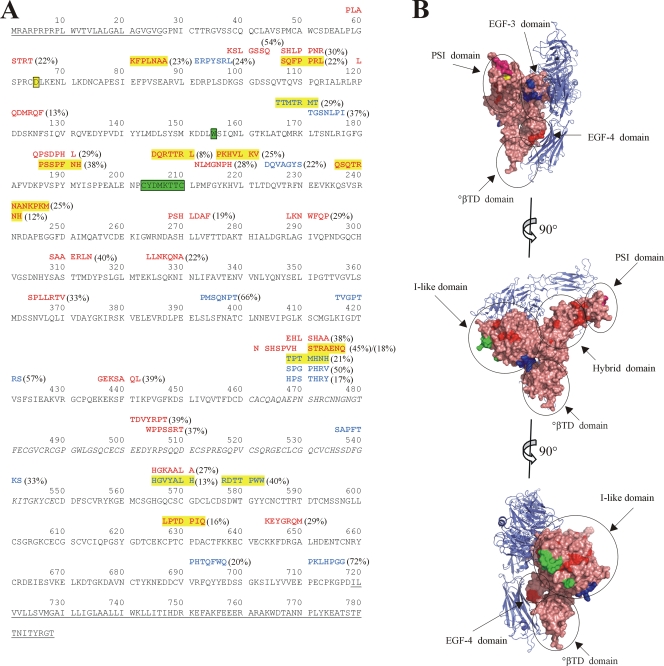
FIG. 1.
Inhibitory peptides identified through phage panning against ANDV show homology to integrin β3. (A) Alignment of phage peptide sequences with P values for integrin β3 pairwise alignment of less than 0.05. Residues comprising the signal peptide, transmembrane, and cytoplasmic domains, which were not included during pairwise alignment, are underlined. Residues 461 to 548, which are missing in the crystal structure, are italicized. Residues involved in the ReoPro binding site are highlighted in green (2). Residue D39 of the PSI domain is highlighted in yellow (19). Peptides are shown above the sequence of integrin β3, with antibody 6C5/D12-eluted sequences shown in blue text and sequences eluted with antibody 6B9/F5 shown in red. Peptide sequences with alignment P values of ≤0.0005 are highlighted in yellow. Percent inhibition of the peptide-bearing phage is shown in parentheses. (B) View of integrin αvβ3 (PDB ID 1U8C [23]). αv is shown in blue ribbon diagram, and β3 is shown in salmon-colored surface representation, with specific domains circled. Residues corresponding to the ReoPro binding site are shown in green, as in panel A, and D39 is shown in yellow. Regions corresponding to 6C5/D12-eluted peptides with P values of ≤0.0005 for alignment with integrin β3 (highlighted in panel A) are shown in blue, and those corresponding to 6B9/F5-eluted peptides with P values of ≤0.0005 for alignment with integrin β3 are shown in red. Alignment of peptide PLASTRT (P value of 0.0040) adjacent to D39 of the PSI domain is shown in magenta. Graphics were prepared using Pymol (DeLano Scientific LLC, San Carlos, CA).