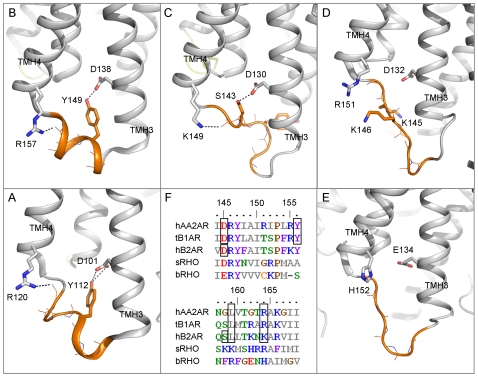
Figure 4. Structural and sequence diversity of ICL2 between the five template GPCRs.
Both A) hAA2AR and B) tB1AR have helical structures (shown in orange) within ICL2. In both of these structures an Arg residue caps the ICL2 helix C-termini and a Tyr sidechain forms a hydrogen bond with an Asp sidechain in TMH3. This combination of constraining hydrogen bond interactions is not observed in C) hB2AR, D) sRHO and E) bRHO where ICL2 is of an irregular coil-like conformation (orange). F) Shows a section of the MSA covering ICL2 and the flanking helix termini. Those residues involved in hydrogen bond interactions in A–E are highlighted in grey boxes. It appears that the presence of both a Tyr at position 156 and an Arg at position 164 as well as the absence of a basic sidechain at position 159 can be used as markers for the presence of helical structures in ICL2.