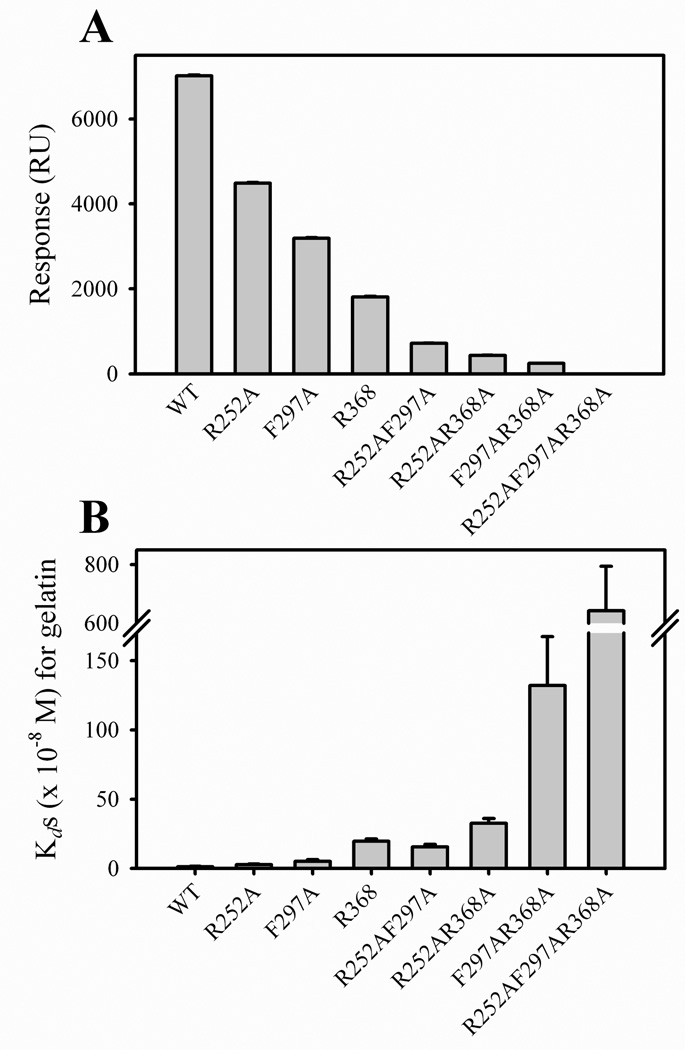
FIGURE 5. Gelatin binding as a function of alanine mutations in collagen binding sites in one, two, and three modules of CBD.
Panel A. The binding of wildtype (WT), as well as single and multi-domain CBD variants to gelatin were measured by surface plasmon resonance (SPR) assays in which CM5 chips were coated with gelatin. Purified CBD variants at 1 µM were passed over the gelatin surfaces in Biacore buffer and the specific binding was expressed in response units (RU). Panel B. Apparent Kds for the interactions of WT and CBD variants with gelatin were measured in plate binding assays and calculated from plots of protein binding versus CBD concentration and using non-linear curve fitting (Sigma Plot).