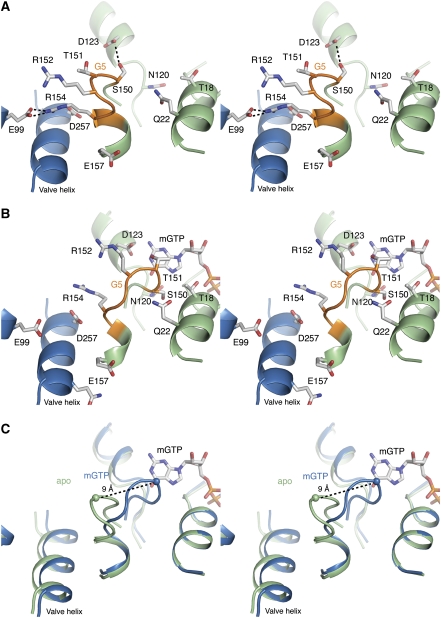
Figure 2.
Stereo views of the G5 motif and mGTP-binding site. (A) Apo state. The G protein is shown in green, with the G5 motif highlighted in orange. The neighbouring protomer is shown in blue. An interprotomeric salt bridge between Arg154 and Glu99, and an intraprotomeric hydrogen bond between Asp123 and Ser150 stabilizes the G5 ‘out' state. (B) mGTP state. Contraction of the G protein after GTP binding leads to a high affinity G5 ‘in' state. In the transition, the salt bridge between Arg154 and Glu99, and hydrogen bond between Asp123 and Ser150, are broken. (C) Superimposition reveals a structural shift of ∼9 Å in the Cα position of Thr151 between the ‘in' and ‘out' conformation.