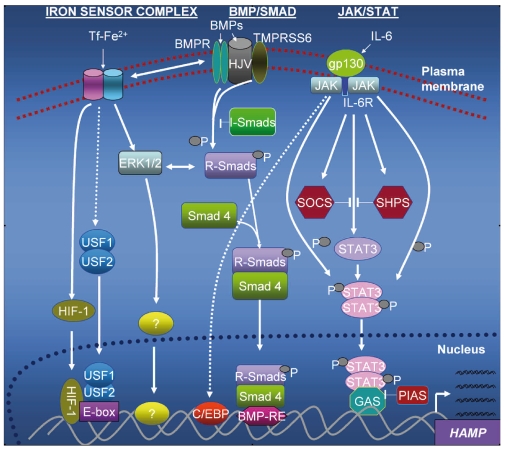
Figure 1.
Regulatory pathways in hepcidin expression. In the BMP/Smad pathway, the binding of BMPs to the BMP receptor induces receptor regulated Smads (R-Smads); following phosphorylation, R-Smads heterodimerize with Smad 4 (common Smad) and co-migrate to the nucleus where they bind to the BMP response elements (BMP-RE) in the hepcidin promoter. R-Smads can be inhibited by inhibitory Smads (I-Smads). An iron sensor complex which may include HFE, TfR2, HJV and TMPRSS6, is regulated by transferrin-bound iron (Tf-Fe). This (hypothetical) complex transmits iron signals via ERK1/2 for activation of a putative iron-responsive transcription factor which binds to the hepcidin promoter or modulates Smad phosphorylation and influences levels of hepcidin expression. Homo- and/or heterodimers of USF1/USF2 compete with HIF-1 for binding to the E-boxes; the signals for this may be generated by the iron sensor complex. The inflammatory (JAK-STAT) pathway engages IL-6 and its receptor, causing phosphorylation of the Janus kinase; this phosphorylates STAT3 which subsequently forms homodimers and translocates to the nucleus where they bind to the interferon γ-activation sequence (GAS) on the hepcidin promoter to drive transcription. The C/EBPs may also be regulated by this pathway (shown with a stippled arrow). The JAK-STAT pathway can be inhibited by the suppressors of cytokine signaling (SOCS), phosphotyrosine phosphatases (SHPS) and PIAS (Protein Inhibitor of Activated STAT). Both the SOCS and SHPs are induced by IL-6 but inhibit JAK-STAT signaling in a negative feed-back loop.