Globin chain synhtesis imbalance is the hallmark of thalassemia syndromes. In this study the authors have crossbred human βIVSII-654 knock-in thalassemic mice with transgenic animals carrying four copies of the human βE-globin gene and analyzed their progeny. The presence of multiple copies of the βE-globin transgene significantly improved the globin chain synthesis ratio and the hematological phenotype of thalassemic mice.
Keywords: thalassemia, transgenic mice, ineffective erythropoiesis, globin chain synthesis
Abstract
Background
β-thalassemia occurs from the imbalanced globin chain synthesis due to the absence or inadequate β-globin chain production. The excessive unbound α-globin chains precipitate in erythroid precursors and mature red blood cells leading to ineffective erythropoiesis and hemolysis.
Design and Methods
In vitro globin chain synthesis in reticulocytes from different types of thalassemic mice was performed. The effect of imbalanced globin chain synthesis was assessed from changes of red blood cell properties including increased numbers of red blood cells vesicles and apoptotic red blood cells, increased reactive oxygen species and decreased red blood cell survival.
Results
The α/β-globin chain ratio in βIVSII-654-thalassemic mice, 1.26±0.03, was significantly higher than that of wild type mice, 0.96±0.05. The thalassemic mice show abnormal hematologic data and defective red blood cell properties. These values were improved significantly in doubly heterozygous thalassemic mice harboring 4 copies of human βE-globin transgene, with a more balanced globin chain synthesis, 0.92±0.05. Moreover, transgenic mice harboring 8 extra copies of the human βE-globin transgene showed inversely imbalanced α/β-globin synthesis ratio, 0.83±0.01, that resulted in a mild β-thalassemia phenotype due to the excessive β-globin chains. The degree of ineffective erythropoiesis also correlated with the degree of imbalanced globin chain synthesis. Bone marrow and splenic erythroid precursor cells of βIVSII-654-thalassemic mice showed increased phosphatidylserine exposure in basophilic and polychromatophilic stages, which was restored to the normal level in doubly heterozygous mice.
Conclusions
Imbalanced α/β-globin chain as a consequence of either reduction or enhancement of β-globin chain synthesis can cause abnormal red blood cell properties in mouse models.
Introduction
Thalassemias, the inherited disorders of hemoglobin synthesis, are characterized by the absence or reduction of globin chain synthesis in human erythroid cells. The defective β-globin synthesis in β-thalassemia leads to an excess of unbound α-globins, which are unable to form a hemoglobin tetramer and precipitate in red blood cell (RBCs) precursors in the bone marrow as well as in their progeny in the peripheral blood.1 This results in defective erythroid precursors, and ineffective erythropoiesis. The subsequent anemia stimulates further erythropoiesis, resulting in proliferation and expansion of the bone marrow. Furthermore the degraded products of unbound α-, β-, or γ-hemoglobin subunits such as heme, hemin, hemichrome and free iron also cause pathological changes in thalassemic red cells leading to oxidative damage of RBC membranes including protein band 3 and 4.1.2,3 They also induce the generation of cytoplasmic reactive oxygen species (ROS) in β-thalassemia RBCs, which is higher than those in normal RBCs.4
These abnormal RBCs lose part of their membranes in the form of spectrin-free vesicles.5 Moreover, oxidative damage of RBC membrane could induce loss of membrane lipid bilayer leading to cell surface externalization of phosphatidylserine (PS), which is characteristic of apoptosis.6 In addition, the studies of erythroid precursors in different thalassemic genotypes demonstrated that erythroid apoptosis was 3–4 times higher than that of normal erythroid cells.7,8
Although the effect of imbalanced globin chain to disease severity has been clarified in individuals carrying different combinations of abnormal globin genes, the environment and genetic backgrounds of these individuals are not homogeneous. Other genetic modifying factors and their interactions with the β-globin gene and the environment can contribute to variable clinical symptoms in patients.9 Here we examined the effect of the imbalanced globin chain synthesis to RBC properties and erythroid apoptosis in mouse model, in which the genetic background and environment are controlled.
In order to clarify the effect of unbound α-globins to RBC properties and erythroid cell death, the mouse models presenting different degrees of globin chain imbalance were generated using human βIVSII-654 knock-in thalassemic mice and transgenic mice carrying four copies of human βE-globin gene.10,11 In this study, crossbreeding between the βIVSII-654 -thalassemic mice and βE-globin transgenic mice gave rise to double heterozygous β-thalassemia mice harboring either four or eight copies of human βE-globin transgene. The resulting double heterozygous mice harboring four copies of human βE-globin transgene that have better balanced globin chain synthesis displayed a markedly improved phenotype compared to βIVSII-654 -thalassemic mice.
Design and Methods
Thalassemic mice models
The heterozygous βIVSII-654 thalassemic mouse (denoted as βm/IVSII-654, or 654) has been developed by gene targeting to replace the two murine adult β-globin genes on chromosome 7 with a copy of the human β-thalassemic gene, βIVSII-654, C→T.11 This thalassemic mouse was kindly provided by the Lineberger Comprehensive Cancer Center and Department of Pharmacology, University of North Carolina, Chapel Hill, NC, USA. The human βE-globin transgenic mice (βm/m; βE4/0, or E4) harboring four copies of the transgene on chromosome 2, while all native murine β-globin genes are intact, were generated by using a 183-kb genomic fragment containing βE mutation in the human β-globin locus.10 All animal protocols were approved by the Mahidol University Animal Care and Use Committee (MU-ACUC). Breeding between the heterozygous βIVSII-654 thalassemic mice and human βE-globin transgenic mice resulted in 3 types of mice carrying different combinations of βIVSII-654 thalassemic gene and βE-globin transgene, denoted as 654/E4 (βm/IVSII-654; βE4/0), 654/E4E4 (βm/IVSII-654; βE4/4), and E4E4 (βm/m; βE4/4). Reverse dot blot hybridization was used to distinguish mice carrying bE- or βIVSII-654-globin.12 The E4 and E4E4 mice were distinguished by breeding these mice with wild type mice and analyzing the segregation pattern of the human βE4-globin gene in offspring. Mice that produce all offspring harboring βE-globin transgene are E4E4. Hematologic data were obtained by using the automated hematology analyzer ADVIA 120 (Bayer, Tarry Town, NY, USA).
Globin chain analysis
Analyses of newly synthesized globin chains were carried out using reticulocyte-enriched cells (20–70%), prepared by collecting mouse tail vein blood in heparinized micro-hematocrit capillary tubes and centrifugation at 12,000 rpm for 20 min at 4°C.13 Packed erythroid cells (50 μL) were incubated in 100 μCi/mL [3H] leucine (Amersham, Buckinghamshire, UK) labeling mix supplemented with 50 μg transferrin for 3 h with periodic shaking at 37°C.13,14 The RBCs were then lysed, and the hemoglobin solution was quantified by cyanmethemoglobin method. The globin chains (100 μg) were separated by electrophoresis on polyacrylamide gels in urea, acetic acid and Triton X-100, and stained with Coomassie Brilliant Blue.15 The α, β and βE bands of each sample were separately sliced, decolorized and eluted from the supporting gel by H2O2. All eluents were heated to 60°C to destroy H2O2 that can cause chemiluminescence. Radioactivity of the newly synthesized globin chains was measured by QuantaSmart™ program for the TriCarb Liquid Scintillation Analyzer (Packard, Merided, CT, USA).16
Ineffective erythropoiesis and apoptosis study
Erythropoiesis was analyzed from bone marrow and spleen samples by flow cytometry. Bone marrow cells were harvested by flushing femur with isolation media containing 2% FBS in Iscove modified Dulbecco medium (IMDM). Single spleen cell suspension was obtained by subsequent passage spleen cells in isolation media through decreasing size needles (18-, 20-, and 23-gauge). Isolated cells were stained with 2 μg/mL anti-mouse TER119-phycoerythrin and 5 μg/mL anti-mouse CD71-fluorescence isothiocyanate (BD Bioscience, San Jose, CA, USA), to identify stages of erythroid differentiation. Cell death defined by phosphatidylserine exposure on erythroid precursors was detected by staining with annexin V-allophycocyanin (Caltag Laboratories, Burlingame, CA, USA).13 Samples were analyzed by a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA, USA) and CellQuest software (Becton Dickinson). Small cell debris was excluded by gating on mononuclear cells on the forward vs. side scatter plot, and erythroid precursors identified as TER119+ CD71+cells.
Oxidative stress status in red blood cells
Reactive oxygen species (ROS) were measured by dichlorofluorescein (DCF) assay modified from Fibach’s protocol. Brifely, 0.2 mM dichlorofluorescein diacetate (DCFH-DA) (Sigma, St. Louis, MO, USA) was added to murine erythrocytes from tail vein (1×106 cell/mL in the ion-free HEPES buffered saline for mouse) and incubated at 37°C for 15 min in a humidified atmosphere of 5% CO2.4 Suspension of 10,000 intact RBCs was incubated with or without freshly prepared 1 mM H2O2, at room temperature and fluorescence intensity was analyzed at 0 (control) and 20 min after incubation by FACSCalibur flow cytometry. The absolute fluorescence intensity of DCF, representing the extent of ROS level in the RBCs, was interpreted on a dot-plot of the FL1 (DCF) and forward scatter (FSC).
Phosphatidylserine exposure on the red blood cell surface
Phosphatidylserine (PS) exposure on the outer surface of RBC membrane was studied by annexin V labeling method. Murine erythrocytes from tail vein were resuspended to 4×106 RBCs/mL and labeled with annexin V-fluorescence isothiocyanate (BD Bioscience) in the dark for 15 min. The samples of 50,000 cells were analyzed by a FACSCalibur flow cytometer.17
Red blood cell vesiculation
The numbers of RBC vesicles in peripheral blood were measured by using quantitative two-color flow cytometry.5 Whole blood was collected from mouse tail vein and RBC membrane specific antigen was stained with 2 μg/mL anti-mouse TER119-phycoerythrin (BD Bioscience). Whereas platelets were labeled with 12.5 μg/mL anti-mouse CD41a-fluorescence isothiocyanate (BD Bioscience). In the same tube a known density of fluorescent TruCount™ bead lyophilized pellets (BD Biosciences) was also added to mouse erythrocytes (1×106 RBCs/mL) in HBSM buffer for 30 min at room temperature. The sample of 100,000 cells was determined by a flow cytometer and RBC vesicles were gated according to their forward/side scatters together with monoclonal antibodies to TER119 and CD41a to discriminate RBC vesicles from platelets. The number of TruCount beads was counted and the absolute number of RBC vesicles was then calculated by the following formula.
Red blood cell survival study
In vivo biotinylation of the entire RBCs was performed by tail vein injection of 200 μL 15 mg/mL EZ-Link Sulfo-NHS-Biotin (Pierce, Rockford, IL, USA).17 A 2–3 μL tail blood sample was obtained weekly by tail vein puncture and the number of biotinylated RBCs was determined by incubation of the blood samples (5×106 cells/mL) with 5μg/mL phycoerythrin-conjugated streptavidin (BD Bioscience), and analyzed by a FACSCalibur flow cytometer. The RBC survival curve was created using SPSS for Windows computer software (Version 16.0, SPSS Inc., Chicago, IL, USA) to demonstrate red cell survival and the duration that biotinylated RBCs reduced to 50% (T1/2, survival half-time).
Statistical analysis
Numerical values were expressed as means±SD. Comparisons of hematologic data and red blood cell properties between different mice genotypes were performed with one-way ANOVA and then Tukey test. Statistical calculations and correlation between α/β-globin ratio and hematologic data and red blood cell properties were performed with SPSS software. A p value less than 0.05 was considered statistically significant.
Results
Imbalanced globin chain synthesis in thalassemic mice
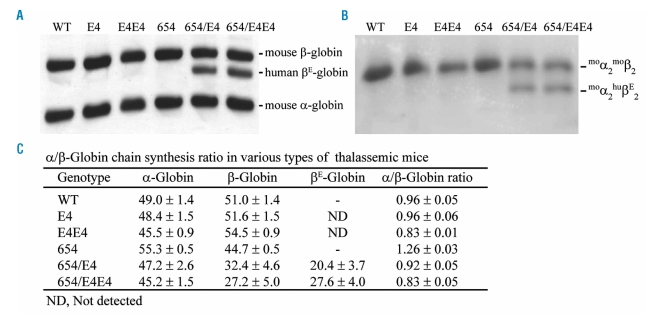
The newly synthesized radioactive-labeled mouse α-and β-globins and human βE-globin chains in reticulocytes were separated in urea, acetic acid and Triton X-100 polyacrylamide gel electrophoresis (Figure 1A). Imbalanced globin chain synthesis with the α/βglobin ratio of 1.26±0.03 were demonstrated in the heterozygous βIVSII-654 thalassemic mice (βm/IVSII-654, 654) (Figure 1C). The ratio was significantly increased in comparison to that of the wild type (0.96±0.05). The human βE-globin transgenic heterozygous mice (βm/m; βE4/0, E4), possessing all of native murine β-globin genes and the integrated human βE-globin transgene, revealed a balance of globin synthesis in the reticulocytes (α/β ratio 0.96±0.06), similar to that of the wild type. Breeding between the 654 thalassemic mice and E4 transgenic mice resulted in double heterozygous mice (βm/hIVSII-654; βE4/0, 654/E4) that have balanced globin chain synthesis with the α/β-globin ratio of 0.92±0.05. The presence of the heterotetramer hemoglobin (muα2/huβE2) in the thalassemic mice was demonstrated using cellulose acetate electrophoresis, which showed that the mouse α-globin chain binds preferentially to mouse β-globin chain than human βE-globin chain (Figure 1B). In human βE-globin transgenic mice (βm/m; βE4/0, or E4) the chimeric hemoglobin was not detected when there is a complete set of mouse β-globin. However, chimeric hemoglobins were detected in double heterozygous mice (βm/IVSII-654; βE4/0 or 654/E4) which produce the decreased mount of mouse β-globin chain.
Figure 1.
Imbalanced globin chain synthesis in thalassemic mice. (A) The globin chain was separated by Triton X-100 acetic acid urea polyacrylamide gel electrophoresis. (B) The cellulose acetate electrophoresis of hemoglobin tetramers in mouse blood. (C) The α/β-globin ratio of wild type (WT), transgenic mice (E4, E4E4) and thalassemic mice (654, 654/E4, 654/E4E4).
Furthermore, crossbreeding between the 654/E4 mice generates homozygous human βE-globin transgenic mice (βm/m; βE4/4, E4E4) which carried all native murine β-globin locus and 8 copies of the integrated human βE-globin transgene, and the thalassemic heterozygous mice harboring 8 copies of human βE-globin transgene (βm/IVSII-654; βE4/4, 654/E4E4). The E4E4 and 654/E4E4 mice showed inverse imbalance of globin synthesis with the α/β-globin ratio of 0.83±0.01 and 0.83±0.05, respectively. This indicated that the E4E4 and 654/E4E4 mice expressed more β-globins than α-globins, which is similar to the mild α-thalassemia.
Ineffective erythropoiesis study in bone marrow and spleen of thalassemic mice
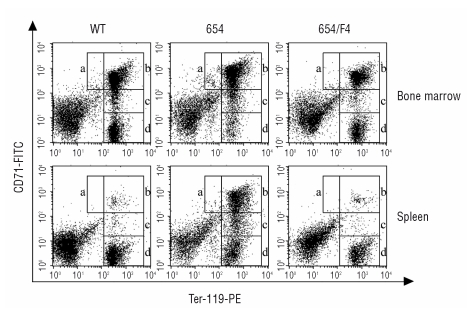
Erythroblast subpopulations in bone marrow and spleen were distinguished by the expression of the transferrin receptor (CD71) and Ter119. The transferrin receptor was expressed at a high level by early erythroid precursors and its level decreased with erythroid maturation. The cell-surface erythroid-specific Ter119 antigen was detected at the intermediate level in proerythroblast and was expressed higher in more mature erythroblasts. Four erythroblast subpopulations could be defined depending on the specific staining characteristics: Ter119medCD71high corresponded to proerythroblasts, Ter119highCD71high corresponded to basophilic erythroblasts, Ter119highCD71med corresponded to late basophilic and polychromatophilic erythroblasts, and Ter119highCD71low corresponded to orthochromatophilic erythroblasts (Figure 2, regions a–d, respectively).
Figure 2.
Flow cytometry dot plots illustrated erythroid precursor subpopulations isolated from bone marrow and spleen of wild type mice (WT), thalassemic mice (654) and thalassemic mice harboring human βE-globin transgene (654/E4). Four regions were delimited by different expression of the Ter119 and CD71. Region a, Ter119medCD71high, corresponded to the proerythroblasts; region b, Ter119highCD71high, corresponded to basophilic erythroblasts; region c, Ter119highCD71med, corresponded to late basophilic and polychromatophilic erythroblasts; region d, Ter119highCD71low, corresponded to orthochromatophilic erythroblasts.
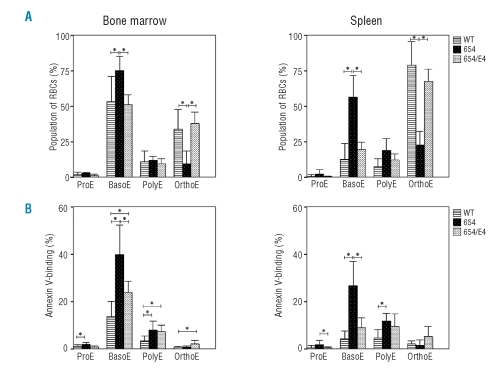
Analysis of marrow and splenic erythroid precursors by Ter119 and CD71 staining showed the increased numbers of basophilic and polychromatic erythroblasts and the decreased orthochromatic erythroblast in the BM and spleen of 654 mice (Figure 2). This implied a blockage in cellular expansion and/or maturational arrest at the basophilic erythroblast stage. Ineffective erythropoiesis, as defined by the low downstream erythroid cell output relative to the precursor production, was significantly observed in the 654 mice, and it was recovered to a similar level of WT in 654/E4 mice (Figure 2, Figure 3A). Significantly enhanced annexin V-positive basophilic erythroblasts demonstrated in thalassemic mice suggested that cell death, which may caused by apoptosis, necrosis or autophagic cell death, was the cause of ineffective erythropoiesis. PS exposure of basophilic erythroblast was recovered in β-thalassemic mice harboring βE transgene (654/E4), which was not significantly different from the wild type (Figure 3B).
Figure 3.
Ineffective erythropoiesis in bone marrow and spleen of wild type (WT, bar with lines), thalassemic mice (654, black bar with dots) and thalassemic mice harboring human βE-globin transgene (654/E4, white bar with dots). (A) The percentages of erythroblasts subpopulation defined by Ter119 and CD71 staining properties. (B) Percent phosphatidylserine exposure cells in the erythroid precursor subpopulations quantified by fluorescence-activated cell-sorting analysis. *p<0.05.
Reduction of red blood cell abnormality and pathology in thalassemic mice carrying human βE-globin transgene
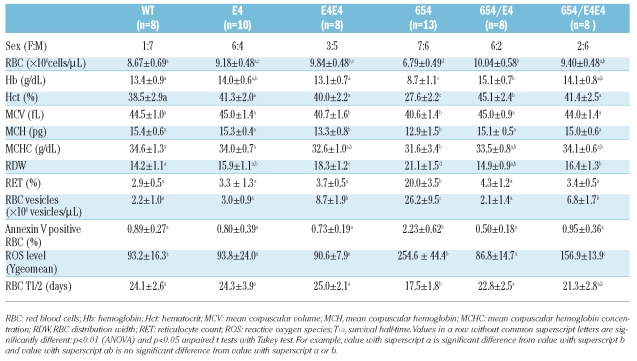
The hematologic data and morphological blood pictures of the wild type and thalassemic mice with different genotypes are shown in Table 1 and Figure 4. The 654 thalassemic mice showed the same clinical features as human thalassemia intermedia with moderate anemia and hepatosplenomegaly. The hematologic parameters showed significant decreases in RBC count, Hb, Hct, MCV, MCH, MCHC and increases in reticulocyte count and RDW. In addition, the peripheral blood smear showed abnormal erythrocytes with hypochromic-microcytic RBCs, schistocytes, target cells, polychromasia and anisocytosis supporting a hemolytic anemia condition.
Table 1.
Hematologic data and red blood cell properties including phosphatidylserine exposure, red blood cell survival, reactive oxygen species and red blood cell vesiculation in thalassemic mice.
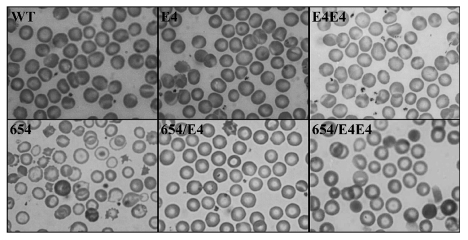
Figure 4.
Red cell morphology of thalassemic mice. Wright-Giemsa staining of peripheral blood smears of wild type (WT), the human βE-globin transgenic heterozygous mice (E4), the homozygote human βE-globin transgenic mice (E4E4), thalassemic mice (654), thalassemic mice harboring human βE-globin transgene (654/E4), the double heterozygous mice harboring human βE-globin transgene 8 copies (654/E4E4).
The general appearance of the RBC morphology in E4 mice was similar to that of the wild type with normochromic normocytic RBCs. The effect of globin synthesis on RBC properties was pronounced in 654/E4 mice. These mice displayed a substantially improved phenotype in comparison to 654 thalassemic mice, which can be demonstrated by the higher hemoglobin level and the normal red cell indices. The normal reticulocyte count in these mice also indicated normal erythropoiesis.
The 654/E4E4 mice showed improvement of hematologic data compared to 654 thalassemic mice but the presence of spherocyte in the blood picture indicated RBC hemolysis. The E4E4 mice also had reduction in MCV, MCH, MCHC and increased in RDW with an anisocytosis blood picture. This data supported the imbalanced globin production in mice with 8 copies of human βE-globin transgene shown in Figure 1.
Changes in red blood cell properties as a consequence of imbalanced globin chain synthesis
In human β-thalassemic RBCs, free heme and α-globin degradation products produce a member of reactive oxygen species (ROS) through Fenton reaction, leading to deterioration of biological activities and functions of bio-molecules.18 The increased ROS level in the RBCs obtained from 654 mice was demonstrated in comparison to the wild type, E4, 654/E4, E4E4 and 654/E4E4 mice (Table 1). The 654 mice revealed significantly higher percentages of PS-exposure RBCs due to the highly oxidative status as predicted. The oxidative damage to RBC membranes could also induce RBCs to lose part of their membranes in the form of spectrin-free RBC vesicles. A significant increase in RBC-derived vesicles in 654 mice (26.2±9.5×104 vesicles/μL) compared to the wild type, E4 and 654/E4 (2.2±1.0×104, 3.0±0.9×104 and 2.1±1.4×104 vesicles/μL, p=4.2×10−9, 9.1×10−13, 6.2×10−13, respectively) was observed. However, the number of RBC-derived vesicles of E4E4 and 654/E4E4 mice was also higher compared with wild type, E4 and 654/E4 mice. This might have resulted from the inversed imbalance of globin synthesis in these mice (Figure 1).
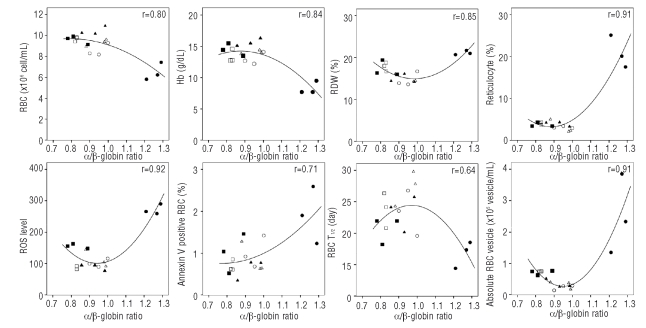
Correlation of imbalanced globin chain synthesis and red blood cells properties
The correlation between the α/β-globin synthesis ratio and all RBC properties including the number of RBC, Hb, Hct, MCV, RDW, reticulocyte count, half-life of RBC survival, PS exposure, ROS levels, absolute number of RBC vesicles of each genotype is shown in Figure 5. The mice that have approximately 0.95 α/β-globin ratio showed normal RBC properties as is shown in WT, E4 and 654/E4 mice. The 654 thalassemic mice have the most imbalanced globin chain synthesis with the α/β-globin ratio of 1.26±0.03 showing the worst hematologic data and RBC properties. However, slightly imbalanced α/β-globin ratio (0.83±0.05 and 0.83±0.01) in 654/E4E4 and E4E4 mice also caused some abnormal RBC indices such as anisocytosis (as demonstrated by increased RDW value) even though other RBC properties of these mice appeared to be normal. This indicated that the imbalanced α/β-globin ratio results in abnormal RBC properties in both decreasing β-globin chain (654) and increasing β-globin chain (E4E4 and 654/E4E4) (Figure 5).
Figure 5.
Correlation of α/β-globin synthesis ratio and RBC properties; RBC count (RBC), hemoglobin concentration (Hb), mean cell volume (MCV), RBC distribution with (RDW), reticulocyte count, ROS level, percent Annexin V positive RBC, number RBC vesicle and T1/2 RBC survival. WT (○), 654 (●), 654/E4 (▴), 654/E4E4 (▪), E4 (▵), E4E4 (□).
The RBC survival half-time (T1/2) of wild type and E4 transgenic mice was not significantly different (24.1±2.6 and 24.3±3.9 days, respectively). Whereas the value in 654 heterozygous mice was significantly decreased (17.5±1.8 days, p=0.00016) and was improved to the same level as that of the wild type in doubly heterozygous 654/E4 mice (22.8±2.5 days). The E4E4 and 654/E4E4 mice also had similar RBC survival half-time to that of the wild type (Table 1).
Discussion
The excess of unbound globin chains that precipitated in erythroid cells liberate iron, bind to membrane, and alter membrane lipids and proteins through oxidative mechanisms, thus causing premature cell destruction. The effect of imbalanced globin chain to disease severity has been demonstrated in thalassemic patients carrying different combinations of abnormal globin gene. However, the environmental and genetic backgrounds of these individuals are not homogeneous, which can contribute to variable clinical symptoms.9 Here we examined the effect of the imbalanced globin chain synthesis in mouse model, in which the genetic background and environment are controlled. The in vitro globin chain synthesis performed in this study showed that the severity of β-thalassemia is directly related to the imbalanced synthesis of the α- and β-globins.
It is noteworthy that the α/β-globin chain ratio in 654 thalassemic mice was not 2.0 as one would expected as in a human β-thalassemia heterozygote. This probably results from the turnover effect. The α/β-globin chain ratio in 654 thalassemic mice, 1.26±0.03, was consistent with previous studies, which showed that α/βglobin ratios of β-thalassemic mice varied between 1.23 and 1.33.13,14 The α/β-globin chain ratio in 654 thalassemic mice was higher than that of wild type mice, 0.96±0.05, and the ratio became more balanced in thalassemic mice harboring 4 copies of human βE-globin transgene, 0.92±0.05. This data indicated that the 4 copies of human βE-globin transgenes express, leads to a more balanced α/β-globin synthesis ratio in 654/E4 thalassemic mice and consequently ameliorates the disease severity caused by excess α-globin chains. The importance of imbalanced globin chain synthesis is also emphasized by the study, which showed that excess α-globin chain synthesis in peripheral blood of β-thalassemia patients correlated with the shortened red cell survival.19 Our result also confirmed the decreased RBC survival in thalassemic 654 mice. Ineffective erythropoiesis along with the decreased survival of erythrocytes in peripheral blood causes anemia.6,20,21
Interestingly, the extra copies of the human βE-globin transgene in E4E4 mice can cause an inversely imbalanced α/βsynthesis ratio, 0.83±0.01. The hematologic data of homozygous mice with 4 copies of the human βE-globin transgenic, E4E4, had the abnormal red cell morphology, reduction of RBC indices, and the increased RDW and RBC vesicles. The data suggested that E4E4 mice have the mild α-thalassemia phenotype due to excess β-globin chain synthesis.
The effect of imbalanced α/βglobin chains was clearly shown in erythroid precursor cells. Analyses of bone marrow and splenic erythroid precursor cells revealed both erythroid expansion and increased PS exposure at basophilic and polychromatophilic erythroblast stages. This finding is consistent with the finding in the hemizygous α- and β-thalassemic mice, which showed maturation arrest in the erythroid precursors after the basophilic erythroblast stage.13,22 Recently ineffective erythropoiesis in β-thalassemia was demonstrated to be the result of the altered cell cycle and decreased cell differentiation as well as apoptosis.22 Study of thalassemic bone marrow progenitor cells also demonstrated that there was a decreased number of mature red blood cells due to an increased level of apoptosis at the polychromatic erythroblast stage.23 Here we showed that the number of PS exposure erythroid precursor cells and ineffective erythropoiesis were reduced in β-thalassemic mice carrying human βE transgene, 654/E4, compared to 654 thalassemic mice, and it was correlated with the more balanced globin chain synthesis in the former.
β-thalassemia patients have a wide range of clinical symptoms from a mild β-thalassemia to a transfusion-dependent disorder. The hemoglobin levels range from 3 to 13 g/dL with an average level of 7.7 g/dL.24 Reduction of α-globin synthesis, through co-inheritance of α-thalassemia, is a well documented background modifier in ameliorating disease severity in β-thalassemia. For example, co-inheritance of α-thalassemia in β-thlassemia/Hb E is associated with milder clinical symptom whereas co-inheritance of triplicated α-globin gene leads to more severe anemia.25–27 Similarly, co-inheritance of α-thalassemia in β-thalassemic mice was also found to ameliorate the thalassemic phenotype, as the reduction of α-globin expression yielded more balanced globin chains.28 Moreover, the reduction of α-globin expression by short-interfering RNA (siRNA) in murine thalassemic primary erythroid cultures was able to restore αβ: -globin mRNA ratios to the balanced wild type levels resulting in the detectable phenotypic correction,29 indicating a potential therapeutic application in the treatment of β-thalassemia.
In conclusion, this study confirms that the different clinical severity in thalassemic mice is due to the level of the imbalanced globin chain synthesis between the α-and non-α-globins. Several features of the thalassemic mice are similar to human β-thalassemia/Hb E patients such as abnormal hematologic parameters, ineffective erythropiosis, and short RBC survival. The expression of human βE-globin transgene compensates for the loss of one set of murine β-globin gene in 654/E4 double heterozygous mice, resulting in normal RBC property compared to those of the 654 mice. Similarly to thalassemic patients, these 654 thalassemia mice also showed similar stages of erythroid apoptosis and ineffective erythropoiesis. Our results also illustrate that the mouse models are suitable for in vivo studies of ineffective erythropoiesis and other pathophysiologies of thalassemias.
Footnotes
Authorship and Disclosures
KS performed the research, analyzed the data and drafted the manuscript. SS contributed to the study design and concept of the study, supervised the research, interpretation of the data. WC performed laboratory analyses. JV, PV, and SF were responsible for study design. PW contributed to the design, concept of the study and revised the manuscript. All authors approved the final version to be published.
The authors reported no potential conflicts of interest.
Funding: this work was supported by Mahidol University Research Grant. KS and PV are supported by RGJ scholarship from the Thailand Research Fund.
References
- 1.Weatherall DJ, Clegg JB. The thalassemia syndromes. Malden, Massachusetts: Blackwell Science; 2001. [Google Scholar]
- 2.Advani R, Rubin E, Mohandas N, Schrier SL. Oxidative red blood cell membrane injury in the pathophysiology of severe mouse beta-thalassemia. Blood. 1992;79:1064–7. [PubMed] [Google Scholar]
- 3.Cappellini MD, Tavazzi D, Duca L, Graziadei G, Mannu F, Turrini F, et al. Metabolic indicators of oxidative stress correlate with haemichrome attachment to membrane, band 3 aggregation and erythrophagocytosis in beta-thalassaemia intermedia. Br J Haematol. 1999;104:504–12. doi: 10.1046/j.1365-2141.1999.01217.x. [DOI] [PubMed] [Google Scholar]
- 4.Amer J, Goldfarb A, Fibach E. Flow cytometric measurement of reactive oxygen species production by normal and thalassaemic red blood cells. Eur J Haematol. 2003;70:84–90. doi: 10.1034/j.1600-0609.2003.00011.x. [DOI] [PubMed] [Google Scholar]
- 5.Pattanapanyasat K, Noulsri E, Fucharoen S, Lerdwana S, Lamchiagdhase P, Siritanaratkul N, et al. Flow cytometric quantitation of red blood cell vesicles in thalassemia. Cytometry B Clin Cytom. 2004;57:23–31. doi: 10.1002/cyto.b.10064. [DOI] [PubMed] [Google Scholar]
- 6.Kuypers FA, Yuan J, Lewis RA, Snyder LM, Kiefer CR, Bunyaratvej A, et al. Membrane phospholipid asymmetry in human thalassemia. Blood. 1998;91:3044–51. [PubMed] [Google Scholar]
- 7.Centis F, Tabellini L, Lucarelli G, Buffi O, Tonucci P, Persini B, et al. The importance of erythroid expansion in determining the extent of apoptosis in erythroid precursors in patients with beta-thalassemia major. Blood. 2000;96:3624–9. [PubMed] [Google Scholar]
- 8.Pootrakul P, Sirankapracha P, Hemsorach S, Moungsub W, Kumbunlue R, Piangitjagum A, et al. A correlation of erythrokinetics, ineffective erythropoiesis, and erythroid precursor apoptosis in thai patients with thalassemia. Blood. 2000;96:2606–12. [PubMed] [Google Scholar]
- 9.Thein SL. Genetic modifiers of the β-haemoglobinopathies. Br J Haematol. 2008;141:357–66. doi: 10.1111/j.1365-2141.2008.07084.x. [DOI] [PubMed] [Google Scholar]
- 10.Jamsai D, Zaibak F, Vadolas J, Voullaire L, Fowler KJ, Gazeas S, et al. A humanized BAC transgenic/knockout mouse model for HbE/β-thalassemia. Genomics. 2006;88:309–15. doi: 10.1016/j.ygeno.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Lewis J, Yang B, Kim R, Sierakowska H, Kole R, Smithies O, et al. A common human β globin splicing mutation modeled in mice. Blood. 1998;91:2152–6. [PubMed] [Google Scholar]
- 12.Winichagoon P, Saechan V, Sripanich R, Nopparatana C, Kanokpongsakdi S, Maggio A, et al. Prenatal diagnosis of β-thalassaemia by reverse dot-blot hybridization. Prenat Diagn. 1999;19:428–35. [PubMed] [Google Scholar]
- 13.Beauchemin H, Blouin MJ, Trudel M. Differential regulatory and compensatory responses in hematopoiesis/erythropoiesis in α-and β-globin hemizygous mice. J Biol Chem. 2004;279:19471–80. doi: 10.1074/jbc.M309989200. [DOI] [PubMed] [Google Scholar]
- 14.Leroy-Viard K, Rouyer-Fessard P, Beuzard Y. Improvement of mouse β-thalassemia by recombinant human erythropoietin. Blood. 1991;78:1596–602. [PubMed] [Google Scholar]
- 15.Alter BP, Goff SC, Efremov GD, Gravely ME, Huisman TH. Globin chain electrophoresis: a new approach to the determination of the Gγ/Aγ ratio in fetal haemoglobin and to studies of globin synthesis. Br J Haematol. 1980;44:527–34. doi: 10.1111/j.1365-2141.1980.tb08706.x. [DOI] [PubMed] [Google Scholar]
- 16.Peerapittayamongkol C, Bernini L, Wilairat P. Presence of αCS-globin on membrane of red cells containing hemoglobin constant spring (CS) J Sci Soc Thailand. 1996;22:117–20. [Google Scholar]
- 17.de Jong K, Emerson RK, Butler J, Bastacky J, Mohandas N, Kuypers FA. Short survival of phosphatidylserine-exposing red blood cells in murine sickle cell anemia. Blood. 2001;98:1577–84. doi: 10.1182/blood.v98.5.1577. [DOI] [PubMed] [Google Scholar]
- 18.Kattamis C, Kattamis AC. Oxidative stress disturbances in erythrocytes of beta-thalassemia. Pediatr Hematol Oncol. 2001;18:85–8. doi: 10.1080/088800101300002900. [DOI] [PubMed] [Google Scholar]
- 19.Vigi V, Volpato S, Gaburro D, Conconi F, Bargellesi A, Pontremoli S. The correlation between red-cell survival and excess of α-globin synthesis in β-thalassemia. Br J Haematol. 1969;16:25–30. doi: 10.1111/j.1365-2141.1969.tb00375.x. [DOI] [PubMed] [Google Scholar]
- 20.Yuan J, Angelucci E, Lucarelli G, Aljurf M, Snyder LM, Kiefer CR, et al. Accelerated programmed cell death (apoptosis) in erythroid precursors of patients with severe β-thalassemia (Cooley’s anemia) Blood. 1993;82:374–7. [PubMed] [Google Scholar]
- 21.Yuan J, Bunyaratvej A, Fucharoen S, Fung C, Shinar E, Schrier SL. The instability of the membrane skeleton in thalassemic red blood cells. Blood. 1995;86:3945–50. [PubMed] [Google Scholar]
- 22.Libani IV, Guy EC, Melchiori L, Schiro R, Ramos P, Breda L, et al. Decreased differentiation of erythroid cells exacerbates ineffective erythropoiesis in β-thalassemia. Blood. 2008;112:875–85. doi: 10.1182/blood-2007-12-126938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathias LA, Fisher TC, Zeng L, Meiselman HJ, Weinberg KI, Hiti AL, et al. Ineffective erythropoiesis in β-thalassemia major is due to apoptosis at the polychromatophilic normoblast stage. Exp Hematol. 2000;28:1343–53. doi: 10.1016/s0301-472x(00)00555-5. [DOI] [PubMed] [Google Scholar]
- 24.Fucharoen S, Winichagoon P, Pootrakul P, Piankijagum A, Wasi P. Variable severity of Southeast Asian β0-thalassemia/Hb E disease. Birth Defects Orig Artic Ser. 1987;23:241–8. [PubMed] [Google Scholar]
- 25.Rees DC. Hemoglobin F and hemoglobin E/β-thalassemia. J Pediatr Hematol Oncol. 2000;22:567–72. doi: 10.1097/00043426-200011000-00025. [DOI] [PubMed] [Google Scholar]
- 26.Sripichai O, Munkongdee T, Kumkhaek C, Svasti S, Winichagoon P, Fucharoen S. Coinheritance of the different copy numbers of α-globin gene modifies severity of β-thalassemia/Hb E disease. Ann Hematol Epub . 2007 Nov 20; doi: 10.1007/s00277-007-0407-2. [DOI] [PubMed] [Google Scholar]
- 27.Winichagoon P, Fucharoen S, Chen P, Wasi P. Genetic factors affecting clinical severity in β-thalassemia syndromes. J Pediatr Hematol Oncol. 2000;22:573–80. doi: 10.1097/00043426-200011000-00026. [DOI] [PubMed] [Google Scholar]
- 28.Voon HP, Wardan H, Vadolas J. Co-inheritance of α- and β-thalassaemia in mice ameliorates thalassaemic phenotype. Blood Cells Mol Dis. 2007;39:184–8. doi: 10.1016/j.bcmd.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Voon HP, Wardan H, Vadolas J. siRNA-mediated reduction of alpha-globin results in phenotypic improvements in β-thalassemic cells. Haematologica. 2008;93:1238–42. doi: 10.3324/haematol.12555. [DOI] [PubMed] [Google Scholar]