The question of the relative efficacy of stem cell sources (bone marrow vs peripheral blood) for sibling allografts still remains, particularly in relation to quality of life. A study of a relatively homogeneous population has confirmed similar outcomes in terms of overall survival, transplant-related mortality or relapse incidence. However acute and chronic graft-vs-host disease showed increases in the peripheral blood group. Possibly as a consequence, although global quality of life did not differ, there was also a significant impairment of role and social functioning in this group.
Keywords: stem cell source, graft-versus-host disease, allogeneic stem cell transplantation
Abstract
Background
Granulocyte colony-stimulating factor mobilized peripheral blood stem cells are increasingly used instead of bone marrow as a stem cell source for transplantation. Whereas this change is almost complete for autologous transplantation, there are some concerns when considering allogeneic transplants.
Design and Methods
We performed a retrospective case-control study including 820 adult patients who had received an allogeneic stem cell transplant from an HLA-identical sibling donor. Quality of life (QoL) was assessed in 150 patients using the EORTC Quality of Life Questionnaire C30 (QLQ-C30).
Results
There were no statistically significant differences in overall survival at ten years (bone marrow: 48.9% vs. peripheral blood stem cells: 39.8%; p=0.621), transplant-related mortality (bone marrow: 28.9% vs. peripheral blood stem cells: 34.4%; p=0.682) or relapse incidence at 9 years (29.4% vs. 35.2%, respectively; p=0.688). Similar outcomes were maintained independently of the phase of the disease. However, multivariate analysis identified a higher incidence of acute graft-versus-host disease grades II-IV (p: 0.023; Hazard ratio [HR]: 1.41; 95% confidence interval [CI]: 1.05–1.89) and grades III-IV (p: 0.006; HR: 1.89; 95% CI: 1.20–2.98), in the peripheral blood stem cells-stem cell transplant group. As previously described, extensive chronic graft-versus-host disease was also more frequent in the peripheral blood stem cells group (28% vs. 15.6%; p<0.001). Patients transplanted with peripheral blood stem cells had significant impairment of role and social functioning.
Conclusions
Although overall survival was not affected by the stem cell source, peripheral blood stem cell transplants were associated with a higher risk of both acute and chronic GvHD. Global quality of life was similar in both groups, but patients transplanted with peripheral blood stem cells showed worse role and social functioning scores, probably related to the increased incidence of chronic graft-versus-host disease.
Introduction
Allogeneic stem cell transplantation (SCT) from an HLA-identical sibling donor remains the treatment of choice for many hematologic malignancies and genetic diseases. The European Group for Blood and Marrow Transplantation (EBMT) recently reported that 9,661 patients received a first allogeneic stem cell transplant in 2006 in Europe, and 71% of these allogeneic transplants were performed with mobilized PBSC.1
PBSC became the preferred source of stem cells for autologous transplantation between 1992 and 1996. However, for allogeneic transplantation the change of stem cell source was delayed three years, due basically to 2 major concerns. The first was the large number of T-lymphocytes infused with PBSC, which could lead to an increased development of acute graft-versus-host disease (aGvHD). The other concern was the long-term safety of the use of G-CSF in healthy donors. Nevertheless, the follow-up of these G-CSF mobilized donors up to six years showed the absence of long-term complications,2 leading to an increased confidence in the procedure. In addition, the first pilot studies and phase II trials demonstrated a faster hematologic recovery, as seen in the autologous setting, with no significant increase in aGvHD.3–5 All these factors led to an increase in the use of G-CSF mobilized PBSC for allogeneic SCT, and now, almost 75% of allogeneic SCT are performed with PBSC.1
However, many studies have shown that the use of PBSC was associated with an increased risk of chronic GvHD (cGvHD).6–8 Moreover, a randomized study performed by the EBMT showed an increased incidence of aGvHD after PBSC transplantation from HLA-identical sibling donors.8 The association between PBSC transplants and aGvHD has been later confirmed by two meta-analyses.9,10
Similarly, the impact of the stem cell source on transplant-related mortality (TRM) and overall survival (OS) in the allogeneic setting is still not clear. Whereas most of the studies have found a similar OS and TRM between BM and PBSC transplants,8,10,11 other authors found an association between the use of PBSC and a lower TRM and better OS.12–14 In contrast, Eapen et al.15 reported an increased TRM and a worse OS in children receiving an allogeneic PBSC transplant from an HLA-identical sibling, when compared with patients receiving allogeneic BM.
In order to detect the relevant differences between both stem cell sources, we conducted a retrospective case-control study to compare the clinical outcome of patients receiving PBSC or BM allogeneic transplantation from an HLA-identical sibling donor.
Design and Methods
Patients
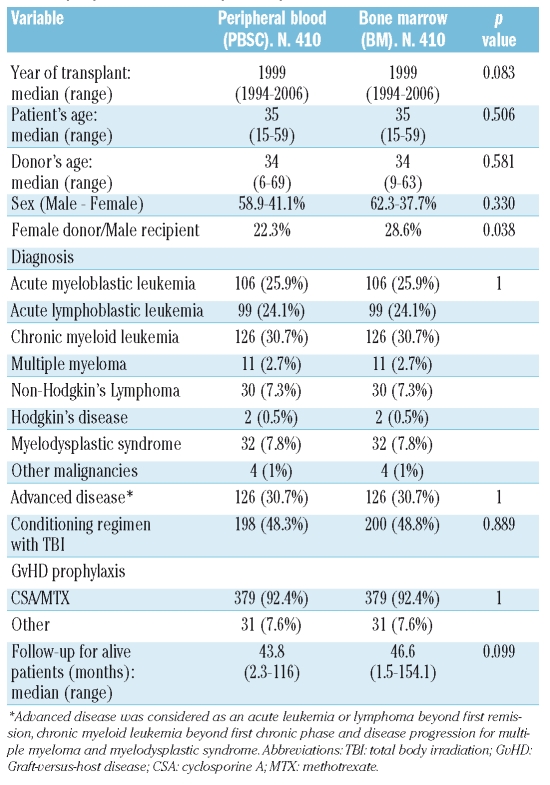
We explored the database from the Spanish Group for hematopoietic transplantation (GETH) that includes 2091 allogeneic transplants performed between 1994 to 2006 from an HLA-identical sibling donor with myeloablative conditioning regimen. This database collects the clinical data of every stem cell transplant performed in Spain, and is maintained by a data manager. As expected, patients transplanted with BM had an earlier date of transplant and a higher percentage of chronic myelogenous leukemia, whereas PBSC transplants were performed later (all were transplanted after 1994) and had a higher percentage of cases transplanted in advanced disease. For this reason, we decided to perform a case–control study, matching for diagnosis and disease status (early versus advanced) (Table 1), and adjusting for the following variables: age at transplantation, year of transplant, conditioning regimen (TBI vs. non-TBI) and GvHD prophylaxis. As a result of this selection, we included in the study 820 patients receiving a first allogeneic stem cell transplant from an HLA-identical sibling donor between 1994 and 2006. The inclusion criteria were: age between 15 and 60 years old, an unmanipulated graft and a myeloablative-conditioning regimen. Patients with aplastic anemia or receiving in vivo T-cell depletion (anti-thymoglobulin or campath) were not included. The patients were transplanted in 27 centers. Four hundred and ten patients received PBSC SCT and 410 controls were transplanted with bone marrow. Baseline characteristics of both groups are shown in Table 1. There were no statistically significant differences between the two groups, except for the combination of a female donor for a male recipient that was more frequent in the BM group. The median follow-up of surviving patients was 44.4 months (range, 1.5–154 months).
Table 1.
Comparison of the clinical characteristics between the bone marrow and the peripheral blood transplanted patients.
Statistical analysis
The homogeneity between the PBSC and BM groups was tested using the χ2 test for qualitative variables and Student’s t-test for continuous variables.
The Kaplan-Meier method was applied for the analysis of OS and disease-free survival (DFS). Curves were compared using the log-rank test. Statistical incidence estimates were used to determine the cumulative incidence of aGVHD, non-relapse mortality and relapse depending on the stem cell source. Death without signs of aGVHD was considered a competing risk in the analysis of aGVHD incidence. Only aGVHD grades II–IV were considered for the analysis of aGVHD incidence. The competing risk for relapse was death in complete remission. Cumulative incidence of non-relapse mortality was calculated after considering death in complete remission as a competing risk. Multivariate Cox regression models using a backward stepwise procedure with the likelihood ratio criterion (inclusion/exclusion criteria: p≤0.05/p>0.10, respectively) were applied to analyze the combined effects of stem cell source and other factors on OS, relapse, and aGVHD. All variables in the univariate analysis with a p value at or below 0.2 were included in the multivariate analysis. Univariate and multivariate logistic regression model were used to evaluate differences in chronic GvHD. p values were two-sided and those lower than 0.05 were considered statistically significant.
Grading of graft-versus-host disease and analysis of outcome
Acute GvHD was graded according to consensus criteria.16 Patients alive 80 days after transplantation with sustained donor engraftment were considered to be evaluable for chronic GvHD.
TRM was defined as death from all causes in the absence of relapse. Once patients were classified as in relapse, they remained so even if they entered a second remission after treatment. Disease-free survival (DFS) was calculated using the time interval from transplantation to either relapse or death in remission, whichever occurred first.
Quality of life assessment
QOL was assessed using the Spanish version of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire, EORTC QLQ-C30, version 3.0 17. The questionnaire consists of 30 items assessing five functional domains (physical, role, emotional, cognitive and social), symptom scales (fatigue, nausea and pain) and six single items (dyspnea, sleep disturbances, appetite loss, constipation, diarrhea, and financial impact of the disease and treatment) and a single global QOL scale. The response options have four categories: not at all, a little, quite a bit or very much, or as a modified visual analog scale going from 1 to 7. All scores are linearly transformed to a 0 to 100 scale. Higher scores on the functioning scales and the global quality of life scale indicate better functioning, while higher scores on the symptom scales reflect more problems. For the analysis, results on the global quality of life and the functioning scales were summarized in very poor (0–20), poor (21–40), intermediate (41–60), good (61–80) and very good (81–100). Symptom scales were summarized as none to slight (0–29), moderate (30–69) and severe (70–100).
Quality of life was assessed in a sample of 150 patients (77 transplanted with PBSC and 73 transplanted with BM) surviving without relapse at least six month after transplantation. These patients were asked to participate in the study when attending their post-transplant clinical control during a six-months period of recruitment. The patients responding in both groups were at similar time points after the transplant. The EORTC QLQ-C30 questionnaires were given directly to the patient by their personal physician. The patients returned the questionnaires either personally or by mail. None of the patients who had been asked to participate refused to collaborate in this study.
The QLQ-C30 responses were scored and analyzed according to the scoring manual provided by the EORTC Study Group on Quality of Life.18
Results
Acute graft-versus-host disease
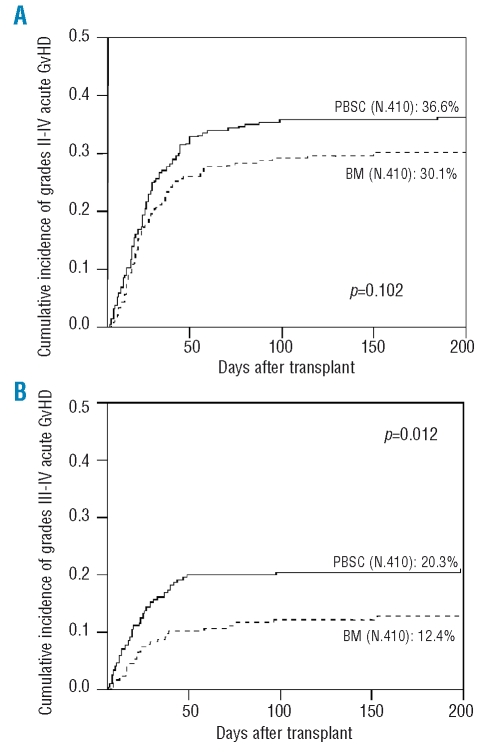
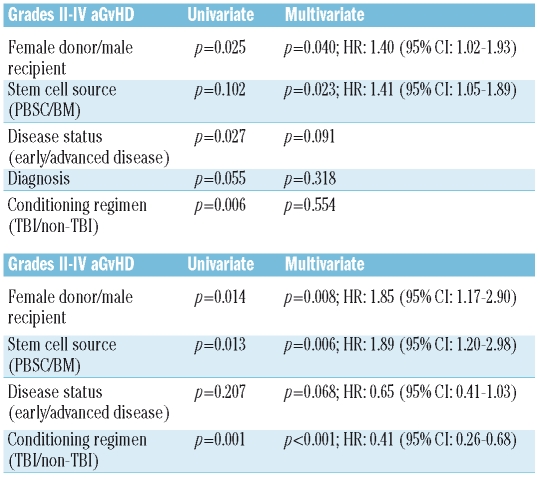
The cumulative incidence of grades II-IV aGvHD was 36.6% for patients receiving PBSC and 30.1% for those receiving BM. This difference was not statistically significant (p=0.102) (Figure 1A). However, in multivariate analysis PBSC was a significant risk factor for grades II-IV aGvHD (p=0.023; HR: 1.41; 95% CI: 1.05–1.89). This difference was greater when only severe (grades III-IV) aGvHD was taken into account, both in univariate (PBSC: 20.3% vs. BM: 12.4%; p: 0.012) (Figure 1B) and in multivariate (HR: 1.89, 95%CI: 1.20–2.98; p=0.006) analysis. Table 2 shows the results of the multivariate analysis.
Figure 1.
Cumulative incidence of grades II-IV (Figure 1A) and III-IV (Figure 1B) acute graft-versus-host disease according to stem cell source.
Table 2.
Multivariate analysis for aGvHD grades II-IV and III-IV. Only variables with a p value <0.2 in the univariate analysis are shown.
No differences in the characteristics of acute GvHD between recipients of BM and PBSC were observed.
Chronic graft-versus-host disease
We found a significantly higher incidence of extensive chronic GvHD after PBSC transplantation (28% vs. 15.6% for BM recipients; HR: 2.11; 95% CI: 1.43–3.11; p<0.001). In multivariate analysis, the use of PBSC was an independent risk factor for extensive chronic GvHD (HR: 2.18, 95%CI: 1.47–3.24; p<0.001).
Relapse
There were no significant differences in relapse incidences between PBSC and BM groups. The relapse incidence at three, five and nine years in the PBSC group was 26.3%, 28.7% and 29.4% vs. 26.8%, 29.7% and 35.2% in the patients treated with BM transplantation, respectively (p=0.688).
The differences in relapse incidences between both stem cell sources were not significant either in the early-disease group (22.4% for patients transplanted with PBSC vs. 21% those receiving BM at three years, 24.5% vs. 23.3% at five years and 25.6% vs. 31.6% at nine years, respectively; p=0.897) nor in the advanced disease group, (35.5% vs. 40.4% at three years, 48.5% vs. 49.2% at five years and 48.5% vs. 49.2% at nine years, respectively; p=0.415).
Transplant-related mortality
The cumulative incidence of mortality due to non-relapse causes was comparable between PBSC and BM: 28.3% vs. 27.2% at three years, 28.7% vs. 27.6% at five years and 34.4% vs. 28.9% at nine years, respectively (p=0.682). The transplant-related mortality (TRM) was also similar for patients with early phase of disease: 22.4% vs. 21% at three years, 24.5% vs. 23.3% at five years and 25.6% vs. 31.6% at nine years, respectively (p=0.897). Patients with advanced disease transplanted with PBSC or BM also showed comparable TRM cumulative incidence (35.5% vs. 40.4% at three years, 48.5% vs. 49.2% at five years and 48.5% vs. 49.2% at nine years, respectively, p=0.415). The day 100 TRM was also similar: PBSC 15% vs. BM 14.5%; p=0.682.
Overall survival
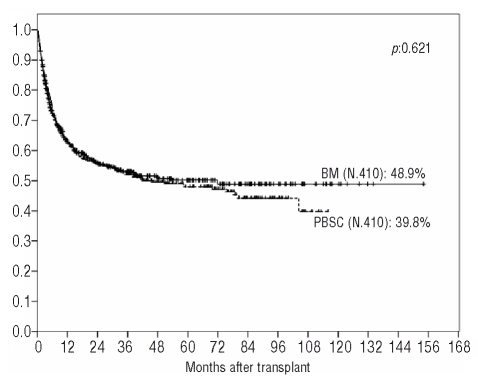
Univariate analysis showed a comparable overall survival for both groups (PBSC: 52% vs. BM: 53.1% at three years; 47.9% vs. 50.2% at five years and 39.8% vs. 48.9% at nine years, respectively; HR: 1.05; 95% CI: 0.86–1.28; p=0.621) (Figure 2). There were also no statistically significant differences between the two groups in patients with early phase disease (PBSC: 61.5% vs. BM: 61% at three years; 57.7% vs. 59.3% at five years and 56.7% vs. 57.4% at nine years, respectively; HR: 0.98; 95% CI: 0.75–1.28; p=0.906) or with advanced disease (PBSC: 29.3% vs. BM: 34.8% at three years, 24.7% vs. 29% at five years and 11.5% vs. 29% at nine years, respectively; HR: 1.19; 95% CI: 0.87–1.61; p=0.265).
Figure 2.
Overall survival according to stem cell source.
Disease-free survival
There were no statistically significant differences in disease-free survival between the PBSC and the BM groups (45.7% vs. 46.1% at three years; 43.4% vs. 42.8% at five years and 36.8% vs. 36.7% at nine years, respectively; HR=1.00; 95% CI, 0.83–1.21; p=0.956). Similar results were obtained when considering only patients with early disease (54.3% vs. 53.4% at three years; 52.3% vs. 50.4% at five years and 50.1% vs. 41.3% at nine years, respectively; HR=0.93; 95% CI, 0.73–1.19; p=0.574) or with advanced disease (25.6% vs. 29.3% at three years; 22.6% vs. 25.2% at five years and 13.2% vs. 25.2% at nine years, respectively; HR=1.15; 95% CI, 0.85–1.55; p=0.341).
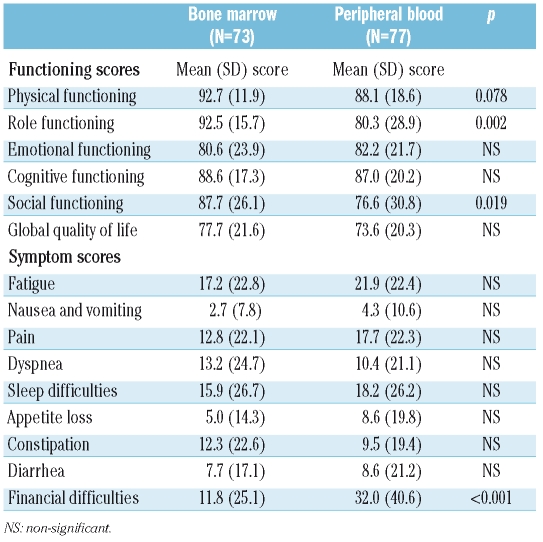
Quality of life assessment
There was no statistical difference in global QoL between patients transplanted with PBSC and those transplanted with BM as a stem cell source: 73% of patients transplanted with PBSC obtained scores indicating a good/very good global health status/QoL, compared with 78.6% for patients receiving BM (p=0.434).
Patients transplanted with PBSC grafts showed statistically significant lower scores in relation to role functioning (work and household activities) and social functioning and a trend towards worse physical functioning. Also, they showed higher scores when measuring financial difficulties related to the treatment and health. The increased financial difficulties are probably due to the greater expenses related with the immunosuppressive drugs required for cGvHD treatment.
Table 3 shows the quality of life scores for both groups after answering the QLQ-C30.
Table 3.
Comparison of quality of life scores between the bone marrow and the peripheral blood groups.
Discussion
Several randomized trials have been performed in order to clarify whether peripheral blood and bone marrow are comparable as a stem cell source for allogeneic transplantation. Despite this, substantial controversy still remains regarding the impact on graft-versus-host-disease (GVHD), mortality or relapse incidence.19 The largest meta-analysis comparing global outcomes of randomized studies found a decrease in the rates of relapse and an increased risk of chronic GVHD for patients receiving PBSC.10 This suggests that chronic GVHD may be an important marker of an active graft-versus-malignancy effect and may be responsible for the reduction in relapse rates and the increase in disease-free survival observed in patients receiving PBSC. We have performed a case-control study with a median follow-up of almost four years for alive patients and a maximum follow-up of ten years. Our results show that the use of PBSC as a stem cell source for allogeneic transplantation from an HLA-identical sibling donor is associated with an increased risk of both acute and chronic GvHD. Whereas the association between PBSC and a higher incidence of cGvHD has been confirmed by almost all the studies, the relation with an increased aGvHD incidence is still controversial. Most of the clinical trials have found a similar incidence of aGvHD after PBSC and BM transplantation. The initial report of the EBMT detected a higher incidence of aGvHD with PBSC,8 but this finding was not confirmed in the three year follow-up study.20 Nonetheless, the two published meta-analyses found a higher incidence of aGvHD after PBSC transplantation,9,10 which is in agreement with our findings.
Interestingly, a lower relapse incidence and a higher DFS have been previously described for patients with advanced disease transplanted with PBSC allografts, suggesting that the higher incidence of chronic GvHD may be an important tool to control the minimal residual disease. However, it has also been suggested that an increased rate of cGvHD-related morbidity and mortality may obviate the long-term benefit. In the present study we found no differences between both stem cell sources in relapse incidence and DFS, even when we focused the analysis on patients transplanted in advanced disease phases. Similarly, we did not observe differences in overall survival at ten years between patients transplanted with BM or PBSC.
Our study offers new data concerning the QoL for long-term survivors. It is well known that extensive chronic GVHD can adversely affect quality of life,21–24 but none of the published randomized trials comparing BM and PBSC collected analyzable data on quality of life. We found that patients receiving PBSC had a worse role and social functioning, and also a trend towards a worse physical functioning. This impairment in functioning scales is probably due to the increased incidence of chronic GvHD after PBSC transplantation. Our results are in agreement with findings reported by Worel et al.21 which described significantly lower physical, role and social scores in patients experiencing chronic GvHD.
We can conclude from our study that, despite obtaining similar long-term survival and DFS, patients receiving PBSC have a higher incidence of both acute and chronic GvHD and worse social and role functioning indicators after quality of life assessment. These data are relevant for clinical practice, and can shift the choice of source from peripheral blood toward bone marrow in a proportion of cases. There are situations where PBSC offer advantages over BM such as weight unbalance between donor and recipient or major ABO incompatibility. However, on most occasions, BM offers a similar survival with less GvHD and a better quality of life. Additional studies to evaluate quality of life with long-term follow-up and a higher number of patients, are needed in order to clarify the role of the different stem cell sources in allogeneic hematopoietic stem cell transplantation. Until these studies clarify the situation, peripheral blood can not be considered equal to or better than bone marrow for an important proportion of patients.
Appendix
List of participating centers: Hospital La Princesa, Madrid; Hospital Morales Meseguer, Murcia; Hospital de la Santa Creu i Sant Pau, Barcelona; Hospital Clínico Universitario, Valencia; Hospital Reina Sofia, Córdoba; Hospital Carlos Haya, Malaga; Hospital Clinic, Barcelona; Hospital Gregorio Marañón, Madrid; Hospital Clínico Universitario, Salamanca; Institut Català d’Oncologia, L’Hospitalet; Institut Català d’Oncologia, Badalona; Hospital Marques de Valdecilla, Santander; Hospital La Fe, Valencia; Hospital Vall d’Hebró, Barcelona; Hospital General Dr Negrin, Gran Canaria; Hospital Son Dureta, Palma de Mallorca; Hospital Central de Asturias, Oviedo; Hospital Puerta de Hierro, Madrid; Hospital La Paz, Madrid; Hospital Nuestra Señora de Aranzazu, San Sebastián; Hospital Virgen de las Nieves, Granada; Hospital 12 de Octubre, Madrid; Hospital Ramón y Cajal, Madrid; Clínica Universitária de Navarra, Pamplona; Complexo Hospitalario Universitário, Santiago de Compostela; Hospital Virgen del Rocío, Sevilla.
Footnotes
Authorship and Dislclosures
DG designed the study, analyzed data and wrote the paper; RdlC, JBN, IE and EC collected data, performed the quality of life studies, revised the article, and gave final approval; AI, AJ-V, CV, CM, DC, SB, DS, CS, JMR and JdlR collected data, revised the article and gave final approval.
The authors reported no potential conflicts of interest.
Funding: this study was financed by grant FIS PI080413 from the Fondo de Investigaciones Sanitarias, Instituto de Salud Carlos III, Ministerio de Sanidad y Consumo.
References
- 1.Gratwohl A, Baldomero H, Frauendorfer K, Rocha V, Apperley J, Niederwieser D. The EBMT activity survey 2006 on hematopoietic stem cell transplantation: focus on the use of cord blood products. Bone Marrow Transplant. 2008;41:687–705. doi: 10.1038/sj.bmt.1705956. [DOI] [PubMed] [Google Scholar]
- 2.Cavallaro AM, Lilleby K, Majolino I, Storb R, Appelbaum FR, Rowley SD, et al. Three to six year follow-up of normal donors who received recombinant human granulocyte colony-stimulating factor. Bone Marrow Transplant. 2000;25:85–9. doi: 10.1038/sj.bmt.1702072. [DOI] [PubMed] [Google Scholar]
- 3.Schmitz N, Dreger P, Suttorp M, Rohwedder EB, Haferlach T, Loffler H, et al. Primary transplantation of allogeneic peripheral blood progenitor cells mobilized by filgrastim (granulocyte colony-stimulating factor) Blood. 1995;85:1666–72. [PubMed] [Google Scholar]
- 4.Bensinger WI, Clift R, Martin P, Appelbaum FR, Demirer T, Gooley T, et al. Allogeneic peripheral blood stem cell transplantation in patients with advanced hematologic malignancies: a retrospective comparison with marrow transplantation. Blood. 1996;88:2794–800. [PubMed] [Google Scholar]
- 5.Przepiorka D, Anderlini P, Ippoliti C, Khouri I, Fietz T, Thall P, et al. Allogeneic blood stem cell transplantation in advanced hematologic cancers. Bone Marrow Transplant. 1997;19:455–60. doi: 10.1038/sj.bmt.1700692. [DOI] [PubMed] [Google Scholar]
- 6.Solano C, Martinez C, Brunet S, Tomás JF, Urbano-Ispizua A, Zuazu J, et al. Chronic graft-versus-host disease after allogeneic peripheral blood progenitor cell or bone marrow transplantation from matched related donors. A case-control study. Spanish Group of Allo-PBT. Bone Marrow Transplant. 1998;22:1129–35. doi: 10.1038/sj.bmt.1701500. [DOI] [PubMed] [Google Scholar]
- 7.Vigorito AC, Azevedo WM, Marques JF, Azevedo AM, Eid KA, Aranha FJ, et al. A randomised, prospective comparison of allogeneic bone marrow and peripheral blood progenitor cell transplantation in the treatment of haematological malignancies. Bone Marrow Transplant. 1998;22:1145–51. doi: 10.1038/sj.bmt.1701510. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz N, Beksac M, Hasenclever D, Bacigalupo A, Ruutu T, Nagler A, et al. Transplantation of mobilized peripheral blood cells to HLA-identical siblings with standard-risk leukemia. Blood. 2002;100:761–7. doi: 10.1182/blood-2001-12-0304. [DOI] [PubMed] [Google Scholar]
- 9.Cutler C, Giri S, Jeyapalan S, Paniagua D, Viswanathan A, Antin JH. Acute and chronic graft-versus-host disease after allogeneic peripheral-blood stem-cell and bone marrow transplantation: a meta-analysis. J Clin Oncol. 2001;19:3685–91. doi: 10.1200/JCO.2001.19.16.3685. [DOI] [PubMed] [Google Scholar]
- 10.Stem Cell Trialists’ Collaborative Group. Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic malignancies: an individual patient data meta-analysis of nine randomized trials. J Clin Oncol. 2005;23:5074–87. doi: 10.1200/JCO.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ringden O, Labopin M, Bacigalupo A, Arcese W, Schaefer UW, Willemze R, et al. Transplantation of peripheral blood stem cells as compared with bone marrow from HLA-identical siblings in adult patients with acute myeloid leukemia and acute lymphoblastic leukemia. J Clin Oncol. 2002;20:4655–64. doi: 10.1200/JCO.2002.12.049. [DOI] [PubMed] [Google Scholar]
- 12.Champlin RE, Schmitz N, Horowitz MM, Chapuis B, Chopra R, Cornelissen JJ, et al. Blood stem cells compared with bone marrow as a source of hematopoietic cells for allogeneic transplantation. IBMTR Histocompatibility and Stem Cell Sources Working Committee and the European Group for Blood and Marrow Transplantation (EBMT) Blood. 2000;95:3702–9. [PubMed] [Google Scholar]
- 13.Couban S, Simpson DR, Barnett MJ, Bredeson C, Hubesch L, Howson-Jan K, et al. A randomized multicenter comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood. 2002;100:1525–31. doi: 10.1182/blood-2002-01-0048. [DOI] [PubMed] [Google Scholar]
- 14.Guardiola P, Runde V, Bacigalupo A, Ruutu T, Locatelli F, Boogaerts MA, et al. Retrospective comparison of bone marrow and granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells for allogeneic stem cell transplantation using HLA identical sibling donors in myelodysplastic syndromes. Blood. 2002;99:4370–8. doi: 10.1182/blood.v99.12.4370. [DOI] [PubMed] [Google Scholar]
- 15.Eapen M, Horowitz MM, Klein JP, Champlin RE, Loberiza FR, Ringden O, et al. Higher mortality after allogeneic peripheral-blood transplantation compared with bone marrow in children and adolescents: the Histocompatibility and Alternate Stem Cell Source Working Committee of the International Bone Marrow Transplant Registry. J Clin Oncol. 2004;22:4872–80. doi: 10.1200/JCO.2004.02.189. [DOI] [PubMed] [Google Scholar]
- 16.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–8. [PubMed] [Google Scholar]
- 17.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The EORTC QLQ-C30: a quality of life instrument for use in international clinical trials in oncology. J Nat Cancer Inst. 1993;85:365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 18.Fayers P, Aarenson N, Bjordal K, Groenvold M, Curran D, Bottomley A. EORTC QLQ-C30 Scoring Manual. EORTC Quality of Life Group; Brussels, Belgium: 2001. [Google Scholar]
- 19.Koca E, Champlin RE. Peripheral blood progenitor cell or bone marrow transplantation: controversy remains. Curr Opin Oncol. 2008;20:220–6. doi: 10.1097/CCO.0b013e3282f5100b. [DOI] [PubMed] [Google Scholar]
- 20.Schmitz N, Beksac M, Bacigalupo A, Ruutu T, Nagler A, Gluckman E, et al. Filgrastim-mobilized peripheral blood progenitor cells versus bone marrow transplantation for treating leukemia: 3-year results from the EBMT randomized trial. Haematologica. 2005;90:643–8. [PubMed] [Google Scholar]
- 21.Worel N, Biener D, Kalhs P, Mitterbauer M, Keil F, Schulenburg A, et al. Long-term outcome and quality of life of patients who are alive and in complete remission more than two years after allogeneic and syngeneic stem cell transplantation. Bone Marrow Transplant. 2002;30:619–26. doi: 10.1038/sj.bmt.1703677. [DOI] [PubMed] [Google Scholar]
- 22.Kiss TL, Abdolell M, Jamal N, Minden MD, Lipton JH, Messner HA. Long-term medical outcomes and quality-of-life assessment of patients with chronic myeloid leukemia followed at least 10 years after allogeneic bone marrow transplantation. J Clin Oncol. 2002;20:2334–43. doi: 10.1200/JCO.2002.06.077. [DOI] [PubMed] [Google Scholar]
- 23.Lee SJ, Kim HT, Ho VT, Cutler C, Alyea EP, Soiffer RJ, et al. Quality of life associated with acute and chronic graft-versus-host disease. Bone Marrow Transplant. 2006;38:305–10. doi: 10.1038/sj.bmt.1705434. [DOI] [PubMed] [Google Scholar]
- 24.Baker KS, Fraser CJ. Quality of life and recovery after graft-versus-host disease. Best Pract Res Clin Haematol. 2008;21:333–41. doi: 10.1016/j.beha.2008.03.002. [DOI] [PubMed] [Google Scholar]