Figure 4. Graphical comparison of the kcat values of FRETN 89-98 alanine-substituted peptides degradation by E. coli Lon.
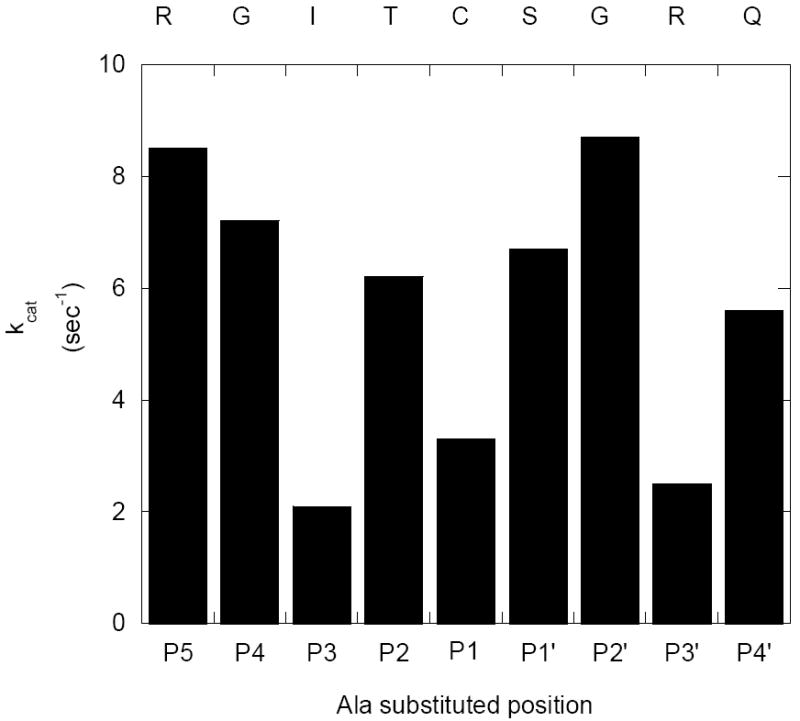
The kcat values for the ATP-dependent cleavage of the Class I peptides by E. coli Lon were determined using the fluorogenic peptidase assay described in methods and materials, in the presence of 150 nM Lon monomer and various concentrations of the indicated peptide at saturating ATP (500 μM). The velocity data were best fit with eq 1 to yield a Hill coefficient of 1.6 and the corresponding kcat and Km values. The kcat values of the respective peptides are compared. The kcat values for the peptides containing Ala substitution at the P3, P1 and P3’ positions are relatively lower than the others. Additional kinetic parameters are summarized in Table 3, under Class I peptides.
