Figure 7. Compare the time course of λN deletion mutants degradation by E. coli Lon.
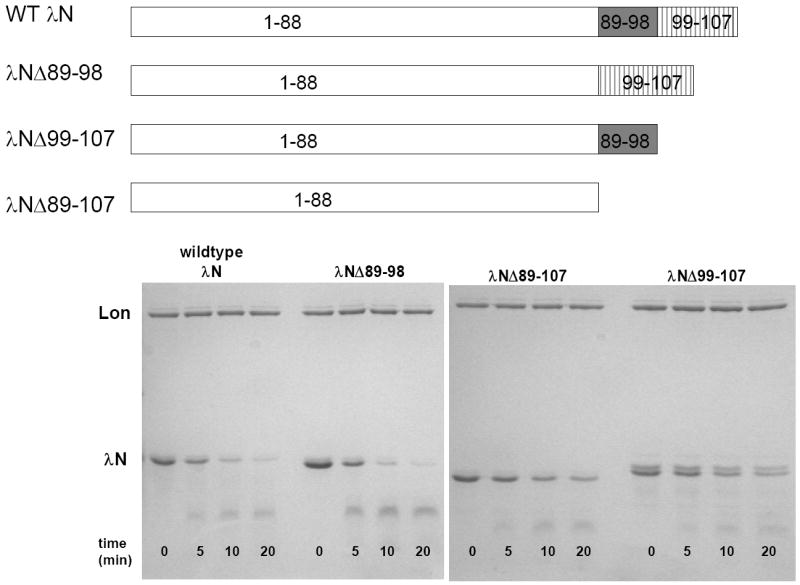
Purified wild-type and truncated λN lacking residues 89-98 (λNΔ89-98), 99-107 (λNΔ99-107) and 89-107 (λNΔ89-107) were digested with E. coli Lon in the presence of 1 mM ATP at the indicated times. The progress of the degradation reactions were monitored by 12.5% SDS-PAGE as described in materials and methods. All three mutants are degraded by Lon, but the wild type and λNΔ89-98 proteins are degraded faster than the λNΔ89-107 and λNΔ99-107 proteins, indicating that the presence of residues 99-107 promotes protein degradation by Lon.
