Abstract
The uterine endometrium, like other mucosal surfaces, is the first line of defence against invading pathogens and must play a stringent role in preventing the establishment of infection in the uterus. However, the bovine endometrium invariably succumbs to bacterial contamination following parturition, most commonly by Escherichia coli. The aim of the present study was to determine whether bovine endometrial cells responded specifically to LPS in vitro and whether they expressed the CD14 and TLR4 transcripts. In addition, since reproductive steroid hormones play an important role in the establishment of infection in the postpartum uterus, the role of hormones in the response to LPS was also investigated. Endometrial stromal and epithelial cells did express CD14 and TLR4 mRNA, and were able to respond to LPS by producing PGE2 and PGF2α respectively, in the absence of immune cells. Furthermore, this functional response was TLR4 mediated since neutralisation of LPS with polymyxin B abrogated the production of PGs. In addition, estradiol and progesterone inhibited the production of PGs by endometrial cells in response to LPS indicating a possible role for steroidal hormones in the response to LPS. Thus, bovine endometrial cells express members of the LPS-receptor complex, are capable of responding to bacterial products but this response is dependent on and influenced by the reproductive hormone milieu.
Keywords: Bacterial, inflammation, reproductive immunology, other animals
Introduction
The uterus is a unique immunological site as it must maintain immunity to constantly invading pathogens yet still remain tolerant to the foetus during pregnancy (1). Like other mucosal surfaces, the endometrium is the first barrier of defence against pathogens and must, therefore, distinguish non-self antigen (2). The ability of the endometrium to carry out this function depends on innate immune receptors recognising conserved sequences on pathogens, known as pathogen-associated molecular patterns. A key group of receptors are the toll-like receptors (TLRs) and nine TLRs are expressed at the mRNA level in the human endometrium with TLR 2, 4, 5 and 9 playing important roles in the recognition and clearance of bacterial infections (2-8).
Female fertility is dependent on the ability of the uterus to counter and eliminate microbial infections. Indeed, there are an estimated 350 million new cases of sexually transmitted diseases each year (www.who.int/topics/sexually_transmitted_infections) with 5% of US women of reproductive age infected and signs ranging from asymptomatic to pelvic inflammatory disease (PID) (9-11). Mammalian fertility is also compromised by PID associated with bacterial infections after parturition, and Bos taurus is a particularly extreme phenotype in which bacterial contamination of the uterus is ubiquitous after parturition (12-15). Bacterial infections in cattle often persist for more than 3 weeks after parturition, causing clinical disease ranging from PID to asymptomatic disease in 15% of animals, resulting in infertility (13). Due to its size and easy accessibility, the bovine uterus provides consequently an excellent model for studying the effects of uterine infection on fertility in mammals.
Amongst the wide range of bacteria that contaminate the bovine postpartum uterus, Escherichia (E.) coli are abundant and are associated with high concentrations of LPS in the uterine lumen and with clinical disease (12, 14, 15). Uterine infection provokes a strong immune response illustrated by the influx of neutrophils into the lumen and increased peripheral plasma concentrations of acute phase proteins (14-16). Indeed, endometrial epithelial cells exhibit immune function and are capable of modifying immune cell recruitment and function at the site of infection (10, 17).
The uterus is influenced by continually changing concentrations of the female sex hormones estradiol and progesterone, which have a profound effect on the establishment of infections (18, 19). For example, in humans, rodents and cattle progesterone suppresses uterine immune function by decreasing the proliferative capacity of lymphocytes, thereby increasing the susceptibility to bacterial infection (18, 19). Furthermore, increased production of progesterone results in decreased production of PGF2α, a pro-inflammatory molecule that enhances neutrophil chemotaxis (18, 20, 21). Conversely, estradiol plays a role in the recruitment of immune cells as studies in rodents have shown that macrophages (MΦ3) are present in large numbers in the endometrium when estradiol levels are high. In addition, IFN-γ secretion is significantly higher in estradiol-supplemented cell cultures from mice. However, estradiol shows biphasic properties as studies in humans have shown that estradiol downregulates cytotoxic activity and suppresses IL-2 production by human peripheral blood lymphocytes suggesting that the effects of estradiol are dependent on estradiol levels (19). However, the effect of estradiol on the immune response to infection in the bovine uterus is less clearly defined and, therefore, our model provides a system to investigate cross-talk between immunity and reproduction.
The objective of the present study was to determine whether bovine endometrial cells respond to LPS and express the mRNA for molecules involved in LPS recognition and binding. Furthermore, since the endometrium is under the continual influence of steroidal hormonal control, which play a role in the establishment of infection, the influence of estradiol and progesterone on the in vitro immune response of stromal and epithelial cells to LPS was investigated.
Materials and Methods
Tissue explants, cell isolation and culture
Bovine uteri were collected from post-pubertal non-pregnant animals with no evidence of genital disease at a local abattoir and kept on ice until further processing in the laboratory. The physiological stage of the reproductive cycle for each genital tract was determined by observation of the ovarian morphology (22). Genital tracts with an ovarian Stage I corpus luteum were used for endometrial culture and only the horn ipsilateral to the corpus luteum was used. Working under sterile conditions, the endometrium was cut into strips and placed into serum-free RPMI-1640 (Sigma) supplemented with 50 IU/ml of penicillin, 50 μg/ml of streptomycin and 2.5 μg/ml of Amphotericin B (Sigma). The strips were then chopped into 1 mm3 pieces using a mechanical tissue chopper (McIlwain Laboratory Engineering, UK) and placed into HBSS (Sigma) (23). 50 mg of tissue were weighed in triplicate for each experimental group within the study and then transferred onto sterile tissue-lined metal grids in 6 well plates (Nunc) with 4.25 ml serum-free RPMI-1640 per well. Plates were incubated at 37°C, 5% CO2 in air, in a humidified incubator (24). Following overnight incubation, supernatants were removed and replaced with fresh media. Endometrial tissue was used for cell isolation as previously described (25, 26) with the following modifications. Briefly, tissue was digested in 25 ml sterile filtered digestive solution, which was made by dissolving 50 mg trypsin III (Roche), 50 mg collagenase II (Sigma), 100 mg BSA (Sigma) and 10 mg DNase I (Sigma) in 100ml HBSS. Following a 1.5 h incubation in a shaking water bath at 37°C, the cell suspension was filtered through a 40 μm mesh (Fisher Scientific) to remove undigested material and the filtrate was resuspended in HBSS containing 10% FBS (Sigma) and 3 μg/ml trypsin inhibitor (Sigma)(Washing medium). The suspension was centrifuged at 100 × g for 10 min and following two further washes in washing medium the cells were resuspended in RPMI-1640 containing 10% FBS, 50 IU/ml of penicillin, 50 μg/ml of streptomycin and 2.5 μg/ml of Amphotericin B. The cells were plated at a density of 1 × 105 cells per 2 ml per well in 24-well plates (Nunc). To obtain separate stromal and epithelial cell populations, the cell suspension was removed 18 h after plating, which allowed selective attachment of stromal cells (25). The removed cell suspension was then replated and incubated allowing epithelial cells to adhere (27). Stromal and epithelial cell populations were distinguished by cell morphology as previously described (25). The culture media was changed every 48 h until the cells reached confluence. All cultures were maintained at 37°C, 5% CO2 in air, in a humidified incubator.
Tissue explant and cell culture challenge
After 24 h culture, tissue explants were challenged with different concentrations of oxytocin (OT3, 3-300 nM, Bachem), LPS (0.03-1 μg/ml, Sigma, E. coli serotype 055.B5) and polymyxin B (2 μg/ml, Sigma) individually or in combination as indicated. Once confluence had been reached, stromal and epithelial cells were challenged with different concentrations of arachadonic acid (AA3, 10-300 μM, Sigma), OT (100 nM), LPS (0.1 −3 μg/ml), heat-killed E. coli (102 −105 CFU/ml, isolated from a case of clinical bovine endometritis associated with pyrexia (14)), polymyxin B (2 μg/ml), 17-β estradiol (3 pg/ml, Sigma) or progesterone (5 ng/ml, Sigma) individually or in combination and for the period of time indicated. Culture supernatants were harvested and frozen prior to cytokine and PG determination.
Prostaglandin radioimmunoassays
Supernatants were analysed for PGE2 and PGF2α by RIA as previously described (28). Samples were diluted in 0.05 M Tris buffer containing 0.1% gelatin and 0.01% sodium azide. Standards and tritiated tracers for the PGs were purchased from Sigma and Amersham International PLC (Amersham, UK), respectively. The antisera were a generous gift from Professor N.L. Poyser (University of Edinburgh, UK) and their cross-reactivities have been reported previously (29). The limit of detection for PGE2 and PGF2α was 2 pg/tube and 1 pg/tube, respectively.
RT – PCR
Total RNA was isolated from cell cultures using the RNeasy Mini Kit (Qiagen, UK) and First Strand cDNA was prepared using SuperScript II RNase H− Reverse Transcriptase (Invitrogen, Life Technologies, UK) according to the manufacturers' protocols. Amplification of cDNA used the following conditions: denaturation for 5 min at 94°C, followed by 30 cycles of 94°C for 30 sec, 54-56°C (depending on primer Tm) for 30 sec and 72°C for 30 sec, followed by a final extension of 5 min at 72°C. Primer combinations were designed using the Mac Vector™ software package and were purchased from MWG (https://ecom.mwgdna.com). Primer sequences are presented in Table 1. All PCR products were sent for sequencing (MRC Geneservice, Camb.) and products showed >98% homology to published sequences on the NCBI database (http://www.ncbi.nlm.nih.gov/blast).
Table I.
Primer sequences: CD45, CD14, TLR4, inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and GAPDH
| Gene | Primer | Sequence | bp |
|---|---|---|---|
| CD45 | Sense | 5′-TGC AAC CGC TCT CTC AAC CAT A-3′ | 845 |
| Anti-sense | 5′-CTT GCT TGG CTT TGC TGG ATC T-3′ | ||
| CD14 | Sense | 5′-GAC GAC GAT TTC CGT TGT GT-3′ | 600 |
| Anti-sense | TGC GTA GCG CTA GAT ATT GGA-3′ | ||
| TLR4 | Sense | 5′-AAC CAC CTC TCC ACC TTG ATA CTG-3′ | 452 |
| Anti-sense | 5′-CCA GCC AGA CCT TGA ATA CAG G-3′ | ||
| iNOS | Sense | 5′-CGG CAT GTG AGG ATC AAA AAC TG-3′ | 270 |
| Anti-sense | 5′-CTC CTT TGT TAC TGC TTC CAC CC-3′ | ||
| COX-2 | Sense | 5′-GGG TGT GAA AGG GAG GAA AGA G-3′ | 128 |
| Anti-sense | 5′-TGA AGT GCT GGG CAA AGA ATG-3′ | ||
| GAPDH | Sense | 5′-GGC GTG AAC CAC GAG AAG TAT AA-3′ | 120 |
| Anti-sense | 5′-CCC TCC ACG ATG CCA AAG T-3′ |
Monoclonal antibodies and flow cytometry
The sources of mouse mAb and isotypes, secondary reagents and methods for flow cytometry have been described in detail (30). The defined surface antigens assessed and mAb used to detect the molecules were: CD14 (CCG33; IgG1) and CD45 (CC171; IgG2a). Isotype matched controls were murine mAb against chicken surface proteins AV20 (Bu-1, IgG1), and AV37 (chicken spleen cell subset, IgG2a) (kindly provided by F. Davison, institute for Animal Health, Compton). Bound mAb were detected with FITC and PE-labelled mouse isotype-specific reagents (Southern Biotechnology Associates (SBA), Birmingham, AL, USA). 10,000 cells were analysed on a FACSAria and immunofluorescent staining was analysed using the FC Express® software (DeNovo Software).
Determination of TNF-alpha (TNFα) and NO
Levels of bioactive TNFα were determined using L929 cells as previously described (31), with the following modifications. L929 cells were cultured in DMEM (Sigma) supplemented with 12.5% FBS, 50 IU/ml penicillin, 50 μg/ml streptomycin and 20 mM HEPES buffer (Sigma). Cells were plated at a density of 2.5 × 104 cells/well in a 96 well plate (Nunc) in 100 μl medium. Cytotoxicity was determined by the colorimetric MTT assay involving the addition of 0.1 μg/ml MTT dye (Sigma) to each well. The cells were then lysed using 100 μl DMSO (Sigma) per well and colour development was read at 560 nm on a Spectra Max 250 (Molecular Devices). The limit of detection was 10 pg/ml and standards were made using recombinant human TNFα (Sigma) and cross-reactivity was determined using recombinant bovine TNFα (kindly provided by Prof C. Howard, Institute for Animal Health, Compton, UK).
NO was measured using the Greiss Reagent System (Promega) and was carried out according to the manufacturer's instructions. The limit of detection was 2.5 μM nitrite.
Statistical analysis
Data were analysed using Mixed Model ANOVA in SAS version 9.1. Results are quoted as mean + SEM, and significance attributed when P<0.05.
Results
Endometrial tissue responds to OT in vitro with the production of prostaglandins
During the normal ovarian cycle, OT release from the ovary or hypothalamus results in the production of PGs from AA by the endometrium (32, 33). To determine whether the endometrial tissues and cells were functional in vitro, the production of PGE2 and PGF2α in response to OT challenge, was measured. Bovine endometrial explants produced both PGE2 and PGF2α in response to OT stimulation (data not shown). Furthermore, stromal and epithelial cells isolated from these explants by tissue digestion released PGE2 and PGF2α respectively in response to OT challenge, though media required supplementation with AA (Fig 1). Interestingly, stromal cells produced little PGF2α and epithelial cells produced no PGE2 (stromal cells: 0.5ng/ml ± 0.1ng/ml PGF2α, and epithelial cells: PGE2 levels below limits of detection). This polarised production of PGs by stromal and epithelial cells supports previous observations (21, 25).
Figure 1.
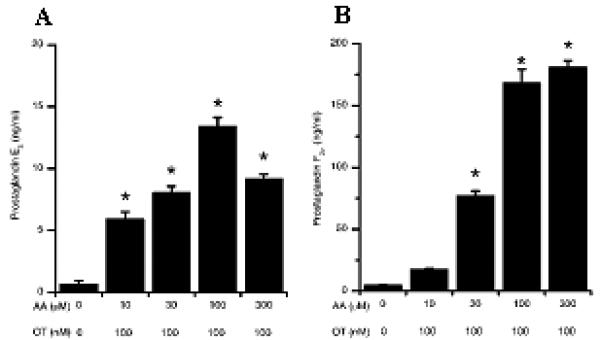
Prostaglandin production by stromal and epithelial cells following OT and AA stimulation. Stromal (A) and epithelial (B) cells were stimulated with OT (100 nM) and AA at the concentrations indicated. After 24 h in culture, supernatants were harvested and prostaglandin production was measured by RIA. *Differences were statistically different at P<0.05 as compared to control. Numerical values are presented as the mean + SEM of three experiments.
Bacterial products drive PG response of endometrial explants
Following parturition, the bovine uterus becomes contaminated with micoorganisms, with E. coli being the most commonly isolated pathogen (14, 15). Since LPS is the main endotoxin of E. coli and a strong activator of the innate immune response, LPS was added to tissue explant cultures and the production of PGE2 and PGF2α was measured. Polymyxin B was used to neutralise LPS and to confirm that the response was LPS mediated (34). Endometrial explants produced increasing amounts of PGE2 and PGF2α in response to LPS in a dose dependent manner (Fig 2). Moreover, this response was dependent on LPS since addition of polymyxin B abrogated the LPS-induced PG production.
Figure 2.
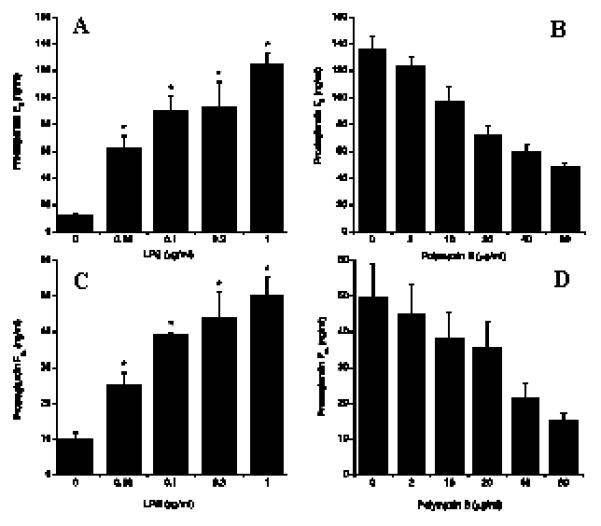
Prostaglandin F2a and E2 production by tissue explants following LPS and polymyxin B challenge. LPS (A and C) and Polymyxin B (B and D) were added at the indicated concentrations. After 24 hours in culture, supernatants were harvested and prostaglandin production was measured by RIA. * Differences were statistically different at P<0.05 as compared to control. Numerical values are presented as the mean + SEM of three experiments.
Endometrial cells produce PG in response to LPS independent of leukocytes, but fail to produce detectable amounts of other immune mediators
Prostaglandins can be produced by a variety of cells, including fibroblasts and MΦ in response to pathogenic stimulation (35). As, it cannot be excluded that other cell types, present in endometrial tissues explants, contributed to the production of PGs, endometrial stromal and epithelial cells cultures were established and analysed for the pan-leukocyte marker CD45 by PCR and flow cytometry. No levels of CD45 mRNA were detected in samples derived from endometrial cell cultures, and this result was confirmed by flow cytometric analysis showing the absence of CD45+ cells (Fig 3). After verification of the absence of immune cells in the endometrial cell preparations, the capacity of these cells to produce PGs in response to pathogenic stimulation was investigated. Both stromal and epithelial cells challenged with heat-killed E. coli produced increased concentrations of PGE2 and PGF2a respectively in response to bacterial challenge (Fig 4) and this response was abrogated by the addition of polymyxin B to the cultures (Fig 4). As LPS was considered the active component the production of PG by stromal and epithelial cells was analysed after incubation of the cells with LPS alone. Both stromal and epithelial cells produced increasing amounts of PGE2 and PGF2a respectively, in a dose dependent manner (Fig 5) and this response was significantly abrogated by neutralising LPS with polymyxin B.
Figure 3.
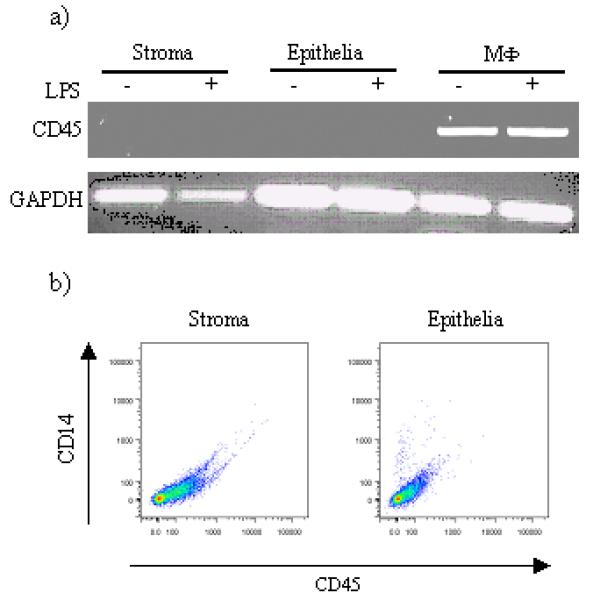
Analysis of CD45 expression by stromal and epithelial cells. A) Cells were stimulated with LPS for 24h, harvested and RNA was isolated as described and the resulting cDNA analysed for the presence of CD45 transcripts using the indicated primer pairs. cDNA from bovine MΦ were used for comparative reason. B) stromal and epithelial cells were stained with antibodies to bovine CD45 and surface expression of these molecules analysed by flow cytometry. A representative staining result is shown (n=3).
Figure 4.
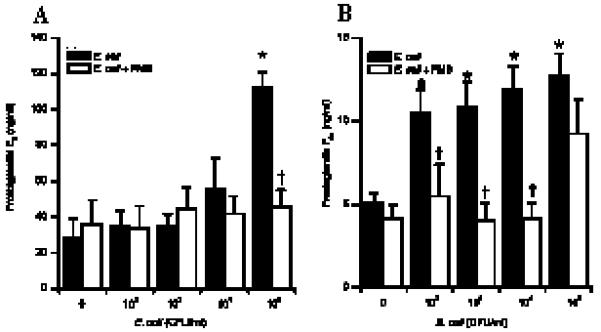
Prostaglandin production by stromal and epithelial cells following E. coli challenge. Stromal (A) and epithelial (B) cells were stimulated with E. coli in the presence (open bar) or absence (closed bar) of polymyxin B (2μg/ml). After 24h in culture, supernatants were harvested and prostaglandin production was measured by RIA. * Differences were statistically different at P<0.05 as compared to control. † Differences were statistically different at P<0.05 as compared to corresponding LPS stimulation. Numerical values are presented as the mean + SEM of three experiments.
Figure 5.
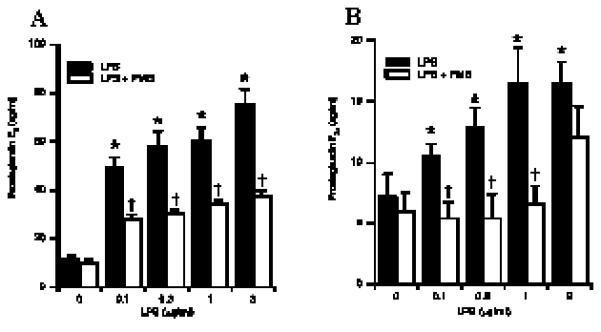
Prostaglandin production by stromal and epithelial cells following LPS challenge. Stromal (A) and epithelial (B) cells were stimulated with LPS in the presence (open bar) or absence (closed bar) of polymyxin B (2μg/ml). After 24h in culture, supernatants were harvested and prostaglandin production was measured by RIA. * Differences were statistically different at P<0.05 as compared to control. † Differences were statistically different at P<0.05 as compared to corresponding LPS stimulation. Numerical values are presented as the mean + SEM of three experiments.
As LPS induces the production of other immune mediators, such as NO and TNFα, by MΦ (36), we consequently analysed levels of NO and TNFα produced by endometrial cell subsets in response to LPS to determine further immune function. However, no detectable levels of NO or TNFα were measured (data not shown). These results show that stromal and epithelial cells respond to pathogenic stimulation, but that this response may be limited or more important for directly stimulating neighbouring cells.
Bovine endometrial cells express members of the LPS-receptor complex
The ability of cells to respond to LPS is dependent on the expression on, and signalling through the receptor complex (5, 37-44). Since stromal and epithelial cells responded to LPS with an increase in PG production (Fig 5), we analysed the expression of two members of the LPS-receptor complex, TLR4 and CD14 by PCR. Using primers designed for the bovine TLR4 and CD14, mRNA transcripts for both were detected in samples derived from endometrial stromal and epithelial cells (Fig 6) indicating that these cells posses the required LPS-receptor complex to respond to LPS.
Figure 6.
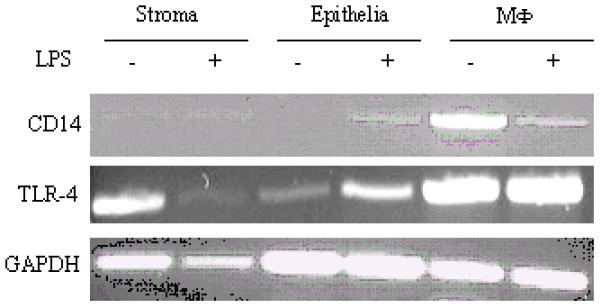
Analysis of CD14 and TLR4 mRNA expression by stromal and epithelial cells. Cells were stimulated with LPS for 24h, harvested and RNA was isolated as described and the resulting cDNA analysed for the presence of CD14 and TLR4 transcripts using the indicated primer pairs. cDNA from bovine MΦ were used for comparative reason.
iNOS and COX-2 are induced in endometrial cells following LPS challenge
Stimulation of the LPS-receptor complex subsequently leads to activation of NFκB and the activation of genes whose products contribute to the inflammatory process, such as the inducible from of nitric oxide synthase (iNOS) leading to the production of NO, and the production of PGs by activating cyclooxygenase enzymes COX-1 and COX-2 (36, 37). However, while COX-1 is expressed constitutively to maintain homeostatic processes, COX-2 is inducible and is involved in the regulation of inflammation (35). Despite the fact that we were unable to detect measurable amounts of NO in stromal and epithelial cell cultures, LPS challenge up-regulate iNOS expression (Fig. 7). Furthermore, an LPS-dependent up-regulation of COX-2 mRNA expression was observed (Fig.7), suggesting that both iNOS and COX-2 are stimulated in stromal and epithelial cells via an LPS-receptor complex pathway involving NFκB.
Figure 7.
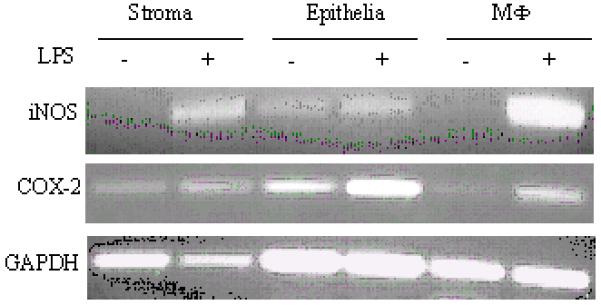
Analysis of iNOS and COX-2 mRNA expression by stromal and epithelial cells. Cells were stimulated with LPS for 24h, harvested and RNA was isolated as described and the resulting cDNA analysed for the presence of iNOS and COX-2 transcripts using the indicated primer pairs. cDNA from bovine MΦ were used for comparative reason.
Sex hormones abrogate LPS-induced PG production by endometrial cell subsets
Several publications indicate that changes in steroid hormone secretion during the estrous cycle change the response of endometrial cells to bacterial stimuli (18, 19). To characterise further the LPS response of stromal and epithelial cells, we investigated the effect of physiological concentrations of ovarian steroid hormones on the response of endometrial cells to LPS. In both cell types, pre-incubation of stromal and epithelial cells with either estradiol or progesterone for 48 hr resulted in a significantly reduced production of PGs in response to LPS challenge (Fig 8).
Figure 8.
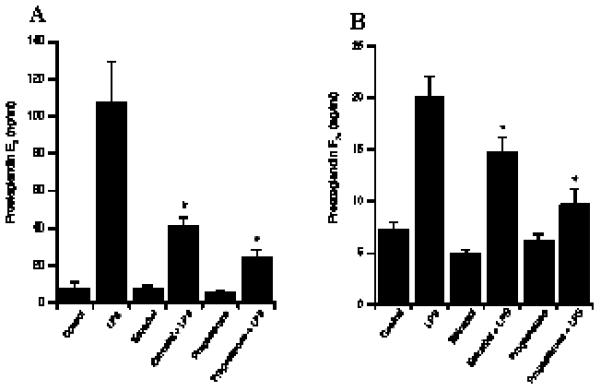
Influence of sex hormones on LPS-stimulated PG production by endometrial cells. Prostaglandin production by stromal (A) and epithelial (B) cells following LPS challenge (1 μg/ml) in the presence of estradiol (3 pg/ml) or progesterone (5 ng/ml). . After 24h in culture, supernatants were harvested and prostaglandin production was measured by RIA. **, *** Differences were statistically different at P<0.01 and P<0.001 respectively as compared to LPS stimulation. Numerical values for stromal and epithelial cells are presented as the mean + SEM of three and nine experiments respectively.
Discussion
The uterus represents an organ within the body, which is constantly exposed to commensals and pathogenic bacteria. Furthermore, uterine infections, either as sexually transmitted diseases or as asymptomatic infections such as PID are on the increase worldwide. However, research on the interactions of uterine cells with invading pathogens is often hampered by the lack of cell-accessibility or established and evaluated models. As the innate immune systems of humans and cattle seem to be closely related, and as uterine infections play an important role in the cattle industry, bovine uterine tissue explants were used as an in vitro model to study the innate immune response to pathogens.
In the present publication, we analysed the ability of bovine tissue explants and cell cultures to produce PGs, as markers of the innate response, in response to OT stimulation. Moreover, the results showed that although tissue explants produced both PGE2 and PGF2α, individual endometrial cell phenotypes showed a more polarised PG production, with stromal cells producing predominantly PGE2 and epithelial cells producing PGF2α. Tissue explants, stromal and epithelial cells produced PGs in response to E. coli and LPS challenge demonstrating that bovine endometrial tissues and cells have the ability to respond to microbial stimulation, indicating that cultures were functional in vitro. This production of PG was LPS mediated as neutralisation of LPS with polymyxin B resulted in the abrogation of PG production. The ability of bovine endometrial cells to respond to LPS suggested the presence of members of the LPS receptor complex, which was confirmed by RT-PCR and our results describe for the first time the expression of CD14 and TLR4 mRNA by bovine endometrial stromal and epithelial cells. Unfortunately, limitations in the availability of bovine TLR4 and CD14 antibodies does not allow us to identify the protein expression of the LPS recognition complex. Interestingly, addition of estradiol and progesterone, hormones involved in the ovarian cycle, to the cultures influenced the production of PGs in response to LPS implicating a direct mechanism of cross-talk between the immune and reproductive system.
Prostaglandins are lipid molecules that are synthesised from AA by cyclooxygenase enzymes, which regulate numerous processes in reproduction as well as immune function and are produced by various cells (32). The role of PGF2α in reproduction is to provide the luteolytic signal required for corpus luteum regression during the oestrus cycle and is produced by epithelial cells in response to oxytocin (21, 32). Conversely, PGE2 is produced by stromal cells and is thought to be responsible for the maintenance of the corpus luteum during pregnancy as well as changes in collagen and vascular permeability (21, 32). The antagonistic functions of PGF2α and PGE2 are further demonstrated in their roles in immunity, where PGF2α has been shown to be pro-inflammatory and PGE2 to be anti-inflammatory (18, 35, 45). In the present study, we challenged bovine endometrial explants to a range of concentrations of LPS found in the plasma of cattle with uterine infections (12, 46). Surprisingly, tissue explant cultures produced both the pro-inflammatory PGF2α and anti-inflammatory PGE2 in response to LPS challenge, although there were higher concentrations of PGE2. However, these explants consist of a variety of cells including resident MΦ, which produce large quantities of PGE2 in response to LPS (35, 47). Therefore, it could not be excluded that the high levels of PGE2 detected in endometrial explant cultures were produced by MΦ. For further investigations, individual endometrial cell subsets were purified and analysed for the absence of CD45+ cells.
The most commonly isolated pathogen from the bovine postpartum uterus is E. coli (12, 15). Using heat-killed E. coli, which we isolated from the uterus of a case of bovine metritis and, was therefore associated with clinical disease as determined by uterine pyrexia (14), we stimulated endometrial stromal and epithelial cells with a range of bacterial concentrations. Endometrial stromal and epithelial cells produced PGs in response to bacterial stimulation and interestingly, epithelial cells responded to bacterial challenge at much lower concentrations compared to stromal cells. This, in part, may be due to the position of stromal and epithelial cells in relation to each other in the endometrium. Since epithelial cells are in contact with the lumen, their location suggests that they may be more sensitive to lower levels of bacteria, ensuring a rapid immune response and clearance of the pathogen. Indeed, epithelial cells are a source of anti-bacterial activity following E. coli or Staphylococcus aureus challenge and therefore their direct contact with the uterine lumen plays an important role in immunity (48). The production of PGs in response to bacterial challenge was in part LPS mediated since addition of polymyxin B abrogated the production of PGs. However, it cannot be excluded that other bacterial products, such as lipoproteins and peptidoglycans, stimulated the production of PGs by endometrial cells via other TLRs. Thus, stromal and epithelial cells were stimulated with the main E. coli endotoxin, LPS.
Interestingly, stromal cell cultures challenged with LPS produced similar concentrations of PGE2 as compared to that of tissue explant cultures. This suggested that bovine endometrial stromal cells were the source of PGE2 in endometrial tissues, capable of producing high levels of PGE2 in response to LPS stimulation. Although epithelial cell cultures produced PGF2α, they produced less PGF2α in response to LPS challenge as compared to tissue explants, suggesting that production of optimal levels of PGF2α was dependent on cross-talk between epithelial cells and other factors found in the bovine endometrium.
The role of PGF2α in immunity is less clearly defined as compared to the role of PGE2 (35, 47). PGE2 results in the down-regulation of Th1 cytokines, such as TNFα, and NO production by MΦ and up-regulates the production of IL-10 (35, 47). The deactivation of MΦ and the down-regulation of pro-inflammatory mediators by PGE2 plays an important role in reproduction as the latter is produced following conception, and therefore plays an important role in suppressing the immune response to the implanting conceptus. In addition, the down-regulation of TNFα and NO by PGE2 may explain the absence of these immune mediators in tissue explant and stromal cell cultures. However, iNOS gene expression was up-regulated following LPS challenge so it cannot be excluded that stromal cells may release NO in concentrations below the detection limits to the assay applied, and the same phenomenon was observed for epithelial cell cultures. Our findings, and those of others (49-51), suggest that despite a clear up-regulation of iNOS mRNA, NO is released only in low concentrations, which may play an important role for the cellular microenvironment. Indeed, NO plays an important role in the production of PGs by bovine uterine stromal and epithelial cells (52, 53).
Stimulation of epithelial cells with LPS resulted in the up-regulation of TLR4, CD14 and COX-2, the enzyme required for the conversion of AA to PGs, which suggested the initiation of a pro-inflammatory response. Since LPS-stimulated stromal cells produced high levels of PGE2, an anti-inflammatory molecule, down-regulation of TLR4 may prevent the cells from further recognising LPS and producing PGE2, thereby preventing suppression of an immune response. These data suggest that a balance in the production of PGs following infection is required such that PGF2α, which encourages a pro-inflammatory response, is prevented from becoming overwhelming by the production of PGE2. Indeed, the balance of PGF2α and PGE2 during infection may have implications on the length of the estrous cycle and is therefore an important factor in cross-talk between immunity and reproduction. Our results therefore showed that endometrial tissue explants, but more importantly pure stromal and epithelial cell cultures, were capable of producing PGs in response to heat-killed E. coli and LPS. Moreover, the response was LPS mediated since neutralisation of LPS with polymyxin B abrogated the production of PGs. Importantly, the balance of PG production following infection determines the length of the reproductive cycle and illustrates a mechanism of cross-talk between immunity and reproduction.
In addition to PGs, the female sex steroids, estradiol and progesterone, have also been shown to play a role in influencing the immune response (18, 19, 45, 48, 54-58). While progesterone suppresses uterine immune responses and can therefore result in the increased susceptibility of the uterus to infection (18, 45, 54, 55), the role of estradiol in infection is dependent on the species, tissue, and concentration of the sex hormone (19). As expected, incubation of stromal and epithelial cells with progesterone resulted in a decrease in the production of PGs following LPS challenge. In addition, incubation of stromal and epithelial cells with estradiol also resulted in a decrease in the production of PGs as previously described (59) although the mechanism of abrogated PG production is not, as yet, known. Others have demonstrated that estradiol inhibits antigen presentation by stromal cells (56). This suggests down-regulation of MHC class I and II, and subsequently estradiol may play a role in the down-regulation of other receptors such as TLRs, which may explain the decreased production of PGs following LPS stimulation. Currently, research in our group is investigating the role of differing concentrations of steroid sex hormones on the response of endometrial cells to LPS stimulation.
Taken together, our results show for the first time that bovine endometrial stromal and epithelial cells respond specifically to LPS stimulation and that this response is possibly based on a pathway involving CD14 and TLR4. Moreover, the response to LPS is dependent on the hormonal milieu, which has important implications for the establishment and treatment of uterine infections. However, it must be noted that the only a single concentration of estradiol and progesterone was used in the present experiment, whereas different concentrations may determine the outcome of cell function (19, 58). Therefore, further work is required to assess the role of cycle-phase specific hormone concentrations on the LPS response of isolated uterine cells.
Acknowledgments
The authors would like to thank Dr. Zhangrui Cheng for technical assistance with RIAs and Dr Andrew Rycroft for expertise in the maintenance of bacterial cultures. We would also like to acknowledge Ally Miller and Kelly Sibley for technical help and the staff of Dawn Cardington abattoir in Bedford for their help with collection of reproductive tracts.
Footnotes
This work was supported by grants from the BBSRC (Grant No. S19795) and The Wellcome Trust (Grant No. 064155).
Abbreviations used in this paper: MΦ, macrophages; OT, oxytocin; AA, arachadonic acid
References
- 1.Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2:656. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 2.Beutler B. Innate immunity: an overview. Mol Immunol. 2004;40:845. doi: 10.1016/j.molimm.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Akira S. Toll receptor families: structure and function. Seminars in Immunology. 2004;16:1. doi: 10.1016/j.smim.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Hoebe K, Janssen E, Beutler B. The interface between innate and adaptive immunity. Nat Immunol. 2004;5:971. doi: 10.1038/ni1004-971. [DOI] [PubMed] [Google Scholar]
- 5.Beutler B, Hoebe K, Du X, Ulevitch RJ. How we detect microbes and repsond to them: the Toll-like receptors and their transducers. J. Leukoc. Biol. 2003;74:479. doi: 10.1189/jlb.0203082. [DOI] [PubMed] [Google Scholar]
- 6.Schaefer TM, Desouza K, Fahey JV, Beagley KW, Wira CR. Toll-like receptor (TLR) expression and TLR-mediated cytokine/chemokine production by human uterine epithelial cells. Immunology. 2004;112:428. doi: 10.1111/j.1365-2567.2004.01898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaefer TM, Fahey JV, Wright JA, Wira CR. Innate Immunity in the Human Female Reproductive Tract: Antiviral Response of Uterine Epithelial Cells to the TLR3 Agonist Poly(I:C) J Immunol. 2005;174:992. doi: 10.4049/jimmunol.174.2.992. [DOI] [PubMed] [Google Scholar]
- 8.Young SL, Lyddon TD, Jorgenson RL, Misfeldt ML. Expression of Toll-like Receptors in Human Endometrial Epithelial Cells and Cell Lines. Am J Reprod Immunol. 2004;52:67. doi: 10.1111/j.1600-0897.2004.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fathalla MF. Reproductive health: a global overview. Ann N Y Acad Sci. 1991;626:1. doi: 10.1111/j.1749-6632.1991.tb37894.x. [DOI] [PubMed] [Google Scholar]
- 10.Quayle AJ. The innate and early immune response to pathogen challenge in the female genital tract and the pivotal role of epithelial cells. J Reprod Immunol. 2002;57:61. doi: 10.1016/s0165-0378(02)00019-0. [DOI] [PubMed] [Google Scholar]
- 11.Butler D. The fertility riddle. Nature. 2004;432:38. doi: 10.1038/432038a. [DOI] [PubMed] [Google Scholar]
- 12.Dohmen MJ, Joop K, Sturk A, Bols PE, Lohuis JA. Relationship between intra-uterine bacterial contamination, endotoxin levels and the development of endometritis in postpartum cows with dystocia or retained placenta. Theriogenology. 2000;54:1019. doi: 10.1016/s0093-691x(00)00410-6. [DOI] [PubMed] [Google Scholar]
- 13.Sheldon IM, Dobson H. Postpartum uterine health in cattle. Anim Reprod Sci. 2004;82-83:295. doi: 10.1016/j.anireprosci.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Sheldon IM, Noakes DE, Rycroft AN, Pfeiffer DU, Dobson H. Influence of uterine bacterial contamination after parturition on ovarian dominant follicle selection and follicle growth and function in cattle. Reproduction. 2002;123:837. [PubMed] [Google Scholar]
- 15.Williams EJ, Fischer DP, Pfeiffer DU, England GC, Noakes DE, Dobson H, Sheldon IM. Clinical evaluation of postpartum vaginal mucus reflects uterine bacterial infection and the immune response in cattle. Theriogenology. 2005;63:102. doi: 10.1016/j.theriogenology.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Zerbe H, Schuberth HJ, Engelke F, Frank J, Klug E, Leibold W. Development and comparison of in vivo and in vitro models for endometritis in cows and mares. Theriogenology. 2003;60:209. doi: 10.1016/s0093-691x(02)01376-6. [DOI] [PubMed] [Google Scholar]
- 17.Philpott DJ, Girardin SE, Sansonetti PJ. Innate immune responses of epithelial cells following infection with bacterial pathogens. Curr Opin Immunol. 2001;13:410. doi: 10.1016/s0952-7915(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 18.Lewis GS. Steroidal regulation of uterine immune defenses. Anim Reprod Sci. 2004;82-83:281. doi: 10.1016/j.anireprosci.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 19.Beagley KW, Gockel CM. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol Med Microbiol. 2003;38:13. doi: 10.1016/S0928-8244(03)00202-5. [DOI] [PubMed] [Google Scholar]
- 20.Del Vecchio RP, Matsas DJ, Inzana TJ, Sponenberg DP, Lewis GS. Effect of intrauterine bacterial infusions and subsequent endometritis on prostaglandin F2 alpha metabolite concentrations in postpartum beef cows. J Anim Sci. 1992;70:3158. doi: 10.2527/1992.70103158x. [DOI] [PubMed] [Google Scholar]
- 21.Asselin E, Goff AK, Bergeron H, Fortier MA. Influence of sex steroids on the production of prostaglandins F2α and E2 and response to oxytocin in cultured epithelial and stromal cells of the bovine endometrium. Biol. Reprod. 1996;54:371. doi: 10.1095/biolreprod54.2.371. [DOI] [PubMed] [Google Scholar]
- 22.Ireland JJ, Murphee RL, Coulson PB. Accuracy of predicting stages of bovine estrous cycle by gross appearance of the corpus luteum. J Dairy Sci. 1980;63:155. doi: 10.3168/jds.S0022-0302(80)82901-8. [DOI] [PubMed] [Google Scholar]
- 23.Leung ST, Cheng Z, Sheldrick EL, Derecka K, Flint AP, Wathes DC. The effects of lipopolysaccharide and interleukins-1alpha, -2 and -6 on oxytocin receptor expression and prostaglandin production in bovine endometrium. J Endocrinol. 2001;168:497. doi: 10.1677/joe.0.1680497. [DOI] [PubMed] [Google Scholar]
- 24.Mann GE. Hormone control of prostaglandin F2α production and oxytocin receptor concentrations in bovine endometrium in explant culture. Domestic Animal Endocrinology. 2001;20:217. doi: 10.1016/s0739-7240(01)00091-1. [DOI] [PubMed] [Google Scholar]
- 25.Fortier MA, Guilbault LA, Grasso F. Specific properties of epithelial and stromal cells from the endometrium of cows. J Reprod Fertil. 1988;83:239. doi: 10.1530/jrf.0.0830239. [DOI] [PubMed] [Google Scholar]
- 26.Cheng Z, Elmes M, Abayasekera DRE, Wathes DC. Effects of conjugated linoleic acid on prostaglandins produced by cells isolated from maternal intercotyledonary endometrium, fetal allantochorion and amnion in late pregnant ewes. Biochimica et Biophysica Acta. 2003;1633:170. doi: 10.1016/s1388-1981(03)00123-9. [DOI] [PubMed] [Google Scholar]
- 27.Kim JJ, Fortier MA. Cell type specificity and protein kinase C dependency on the stimulation of prostaglandin E2 and prostaglandin F2 alpha production by oxytocin and platelet-activating factor in bovine endometrial cells. J Reprod Fertil. 1995;103:239. doi: 10.1530/jrf.0.1030239. [DOI] [PubMed] [Google Scholar]
- 28.Cheng Z, Robinson RS, Pushpakumara PG, Mansbridge RJ, Wathes DC. Effect of dietary polyunsaturated fatty acids on uterine prostaglandin synthesis in the cow. J Endocrinol. 2001;171:463. doi: 10.1677/joe.0.1710463. [DOI] [PubMed] [Google Scholar]
- 29.Poyser NL. Effects of various factors on prostaglandin synthesis by the guinea-pig uterus. J Reprod Fertil. 1987;81:269. doi: 10.1530/jrf.0.0810269. [DOI] [PubMed] [Google Scholar]
- 30.Werling D, Hope JC, Chaplin P, Collins RA, Taylor G, Howard CJ. Involvement of caveolae in the uptake of respiratory syncytial virus antigen by dendritic cells. J Leukoc Biol. 1999;66:50. doi: 10.1002/jlb.66.1.50. [DOI] [PubMed] [Google Scholar]
- 31.Trost LC, Lemasters JJ. A cytotoxicity assay for tumor necrosis factor employing a multiwell fluorescence scanner. Anal Biochem. 1994;220:149. doi: 10.1006/abio.1994.1311. [DOI] [PubMed] [Google Scholar]
- 32.Poyser NL. The control of prostaglandin production by the endometrium in relation to luteolysis and menstruation. Prostaglandins Leukot Essent Fatty Acids. 1995;53:147. doi: 10.1016/0952-3278(95)90115-9. [DOI] [PubMed] [Google Scholar]
- 33.Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271:33157. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs DM, Morrison DC. Inhibition of the mitogenic response to lipopolysaccharide (LPS) in mouse spleen cells by polymyxin B. J Immunol. 1977;118:21. [PubMed] [Google Scholar]
- 35.Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 36.Janeway C, Travers P. Immunobiology. Garland Publishing; New York: 2001. [Google Scholar]
- 37.Takeda K, Akira S. TLR signaling pathways. Seminars in Immunology. 2004;16:3. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Nagai Y, Akashi S, Nagafuku M, Ogata M, Iwakura Y, Akira S, Kitamura T, Kosugi A, Kimoto M, Miyake K. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3:667. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- 39.Abreu MT, Arditi M. Innate immunity and toll-like receptors: clinical implications of basic science research. J Pediatr. 2004;144:421. doi: 10.1016/j.jpeds.2004.01.057. [DOI] [PubMed] [Google Scholar]
- 40.Miyake K. Endotoxin recognition molecules, Toll-like receptor 4-MD-2. Seminars in Immunology. 2004;16:11. doi: 10.1016/j.smim.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 41.Pasare C, Medzhitov R. Toll-like receptors and acquired immunity. Seminars in Immunology. 2004;16:23. doi: 10.1016/j.smim.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 42.Honstettre A, Ghigo E, Moynault A, Capo C, Toman R, Akira S, Takeuchi O, Lepidi H, Raoult D, Mege JL. Lipopolysaccharide from Coxiella burnetii Is Involved in Bacterial Phagocytosis, Filamentous Actin Reorganization, and Inflammatory Responses through Toll-Like Receptor 4. J Immunol. 2004;172:3695. doi: 10.4049/jimmunol.172.6.3695. [DOI] [PubMed] [Google Scholar]
- 43.Dunzendorfer S, Lee HK, Soldau K, Tobias PS. TLR4 is the signaling but not the lipopolysaccharide uptake receptor. J Immunol. 2004;173:1166. doi: 10.4049/jimmunol.173.2.1166. [DOI] [PubMed] [Google Scholar]
- 44.O'Neill LA. TLRs: Professor Mechnikov, sit on your hat. Trends Immunol. 2004;25:687. doi: 10.1016/j.it.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 45.Lewis GS. Steroidal regulation of uterine resistance to bacterial infection in livestock. Reprod Biol Endocrinol. 2003;1:117. doi: 10.1186/1477-7827-1-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mateus L, Lopes da Costa L, Diniz P, Ziecik AJ. Relationship between endotoxin and prostaglandin (PGE2 and PGFM) concentrations and ovarian function in dairy cows with puerperal endometritis. Anim Reprod Sci. 2003;76:143. doi: 10.1016/s0378-4320(02)00248-8. [DOI] [PubMed] [Google Scholar]
- 47.Stenson WF, Parker CW. Prostaglandins, macrophages, and immunity. J Immunol. 1980;125:1. [PubMed] [Google Scholar]
- 48.Fahey JV, Rossoll RM, Wira CR. Sex hormone regulation of anti-bacterial activity in rat uterine secretions and apical release of anti-bacterial factor(s) by uterine epithelial cells in culture. J Steroid Biochem Mol Biol. 2005;93:59. doi: 10.1016/j.jsbmb.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 49.Yoshiki N, Kubota T, Aso T. Expression and localization of inducible nitric oxide synthase in human non-pregnant and early pregnant endometrium. Mol Hum Reprod. 2000;6:283. doi: 10.1093/molehr/6.3.283. [DOI] [PubMed] [Google Scholar]
- 50.Yoshiki N, Kubota T, Matsumoto Y, Aso T. Expression of inducible nitric oxide synthase in human cultured endometrial stromal cells. Mol Hum Reprod. 1999;5:353. doi: 10.1093/molehr/5.4.353. [DOI] [PubMed] [Google Scholar]
- 51.Khorram O, Garthwaite M, Magness RR. Endometrial and myometrial expression of nitric oxide synthase isoforms in pre- and postmenopausal women. J Clin Endocrinol Metab. 1999;84:2226. doi: 10.1210/jcem.84.6.5759. [DOI] [PubMed] [Google Scholar]
- 52.Woclawek-Potocka I, Deptula K, Bah MM, Lee HY, Okuda K, Skarzynski DJ. Effects of nitric oxide and tumor necrosis factor-alpha on production of prostaglandin F2alpha and E2 in bovine endometrial cells. J Reprod Dev. 2004;50:333. doi: 10.1262/jrd.50.333. [DOI] [PubMed] [Google Scholar]
- 53.Skarzynski DJ, Miyamoto Y, Okuda K. Production of prostaglandin f(2alpha) by cultured bovine endometrial cells in response to tumor necrosis factor alpha: cell type specificity and intracellular mechanisms. Biol Reprod. 2000;62:1116. doi: 10.1095/biolreprod62.5.1116. [DOI] [PubMed] [Google Scholar]
- 54.Kaushic C, Murdin AD, Underdown BJ, Wira CR. Chlamydia trachomatis infection in the female reproductive tract of the rat: influence of progesterone on infectivity and immune response. Infect Immun. 1998;66:893. doi: 10.1128/iai.66.3.893-898.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaushic C, Zhou F, Murdin AD, Wira CR. Effects of estradiol and progesterone on susceptibility and early immune responses to Chlamydia trachomatis infection in the female reproductive tract. Infect Immun. 2000;68:4207. doi: 10.1128/iai.68.7.4207-4216.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wira CR, Rossoll RM. Oestradiol regulation of antigen presentation by uterine stromal cells: role of transforming growth factor-beta production by epithelial cells in mediating antigen-presenting cell function. Immunology. 2003;109:398. doi: 10.1046/j.1365-2567.2003.01670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hong M, Zhu Q. Macrophages are activated by 17beta-estradiol: possible permission role in endometriosis. Exp Toxicol Pathol. 2004;55:385. doi: 10.1078/0940-2993-00335. [DOI] [PubMed] [Google Scholar]
- 58.Chao TC, Chao HH, Chen MF, Greager JA, Walter RJ. Female sex hormones modulate the function of LPS-treated macrophages. Am J Reprod Immunol. 2000;44:310. doi: 10.1111/j.8755-8920.2000.440511.x. [DOI] [PubMed] [Google Scholar]
- 59.Kimmins S, Lim HC, Parent J, Fortier MA, MacLaren LA. The effects of estrogen and progesterone on prostaglandins and integrin beta 3 (beta3) subunit expression in primary cultures of bovine endometrial cells. Domest Anim Endocrinol. 2003;25:141. doi: 10.1016/s0739-7240(03)00015-8. [DOI] [PubMed] [Google Scholar]


