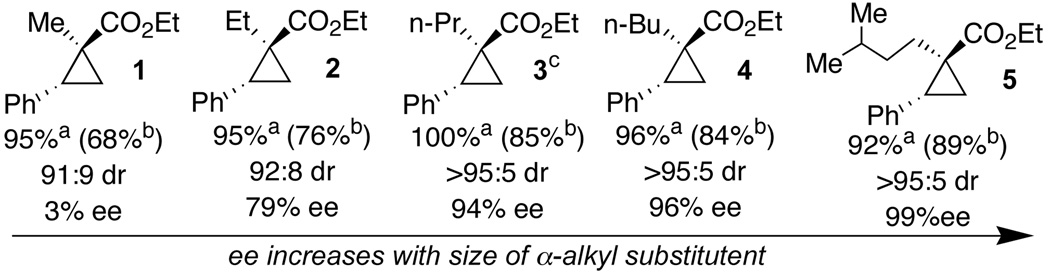
Scheme 1.
Effects of α-alkyl substitution on stereoselectivity
In general, reactions were carried out in hexane at −78 °C with 0.5 mol% Rh2(S-PTTL)4 a Isolated yield with 3 equiv. diazo compound and limiting alkene. bIsolated yield with 1.1 equiv of alkene and limiting diazo compound. cThe yield and ee of 3 dropped in reactions carried out with 3.0 equiv diazoester at 0 °C (54%, 88% ee) and 25 °C (43%, 85% ee).