Fig. 3.
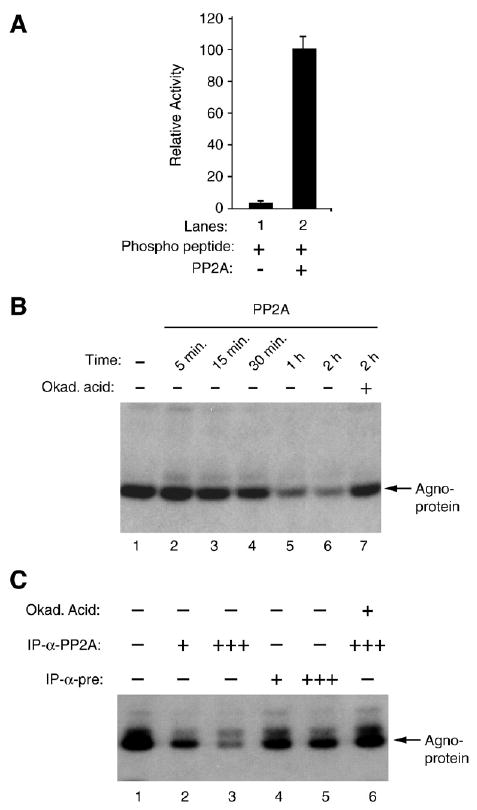
PP2A dephosphorylates agnoprotein. (A) Enzymatic activity of purified PP2A on authentic substrate was measured according to manufacturer's recommendations (Upstate) and expressed as relative activity compared to without enzyme in the reaction. (B) Time course activity of PP2A on phosphorylated agnoprotein. PKC-phosphorylated GST–agnoprotein was equally distributed into reaction tubes, subjected to dephosphorylation by purified PP2A at different time points and analyzed by autoradiography. Okadaic acid was used as a specific inhibitor of PP2A in the reaction (lane 7). (C) PKC-phosphorylated GST–agnoprotein was also subjected to dephosphorylation by immunoprecipitated-PP2A and analyzed by autoradiography as described in Materials and methods. PP2A was immunoprecipitated (IP) from whole cell extracts prepared from SVG-A cells (500 μg) using either α-pre (2 μg) or α-PP2A C antibody (2 μg) prior to dephosphorylation reaction. Immunoprecipitated PP2A was split into 4 equal portions. One (+) or three (+++) portions of immunoprecipitated PP2A were used in the reactions as indicated. In lane 6, okadaic acid (Okad. Acid) was used to inhibit the dephosphorylation reaction.
