Fig. 4.
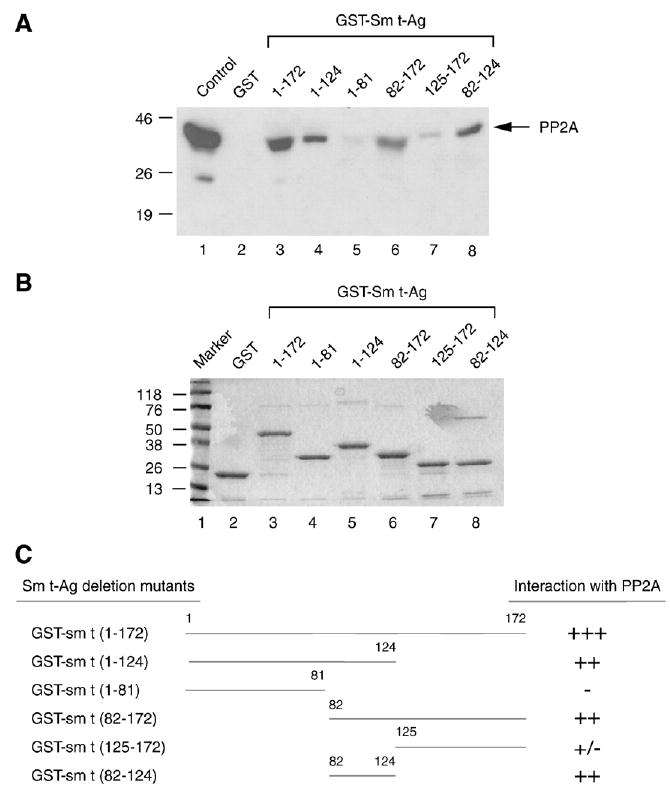
The PP2A interaction domain of the Sm t-Ag localizes to its middle portion. (A) Sm t-Ag and its deletion mutants were expressed in bacteria as fusion proteins and immobilized on glutathione-S-transferase (GST) beads. Whole cell lysates prepared from U-87MG cells were incubated with either GST–Sm t-Ag or its deletion mutants for 2 h. The columns were extensively washed with binding buffer as described in Materials and methods and the proteins retained in the column were fractionated by SDS-10% PAGE and analyzed by Western blotting with an antibody directed against PP2A. (B) Analysis of GST, GST–Sm t-Ag and the deletion mutants of Sm t-Ag by SDS-12% PAGE. (C) Schematic representation of Sm t-Ag and its deletion mutants. The relative binding activity of Sm t-Ag and its deletion mutants is represented by + or − signs. +++, very strong binding; ++, strong binding; +, binding; +/−, weak binding and −, no binding.
